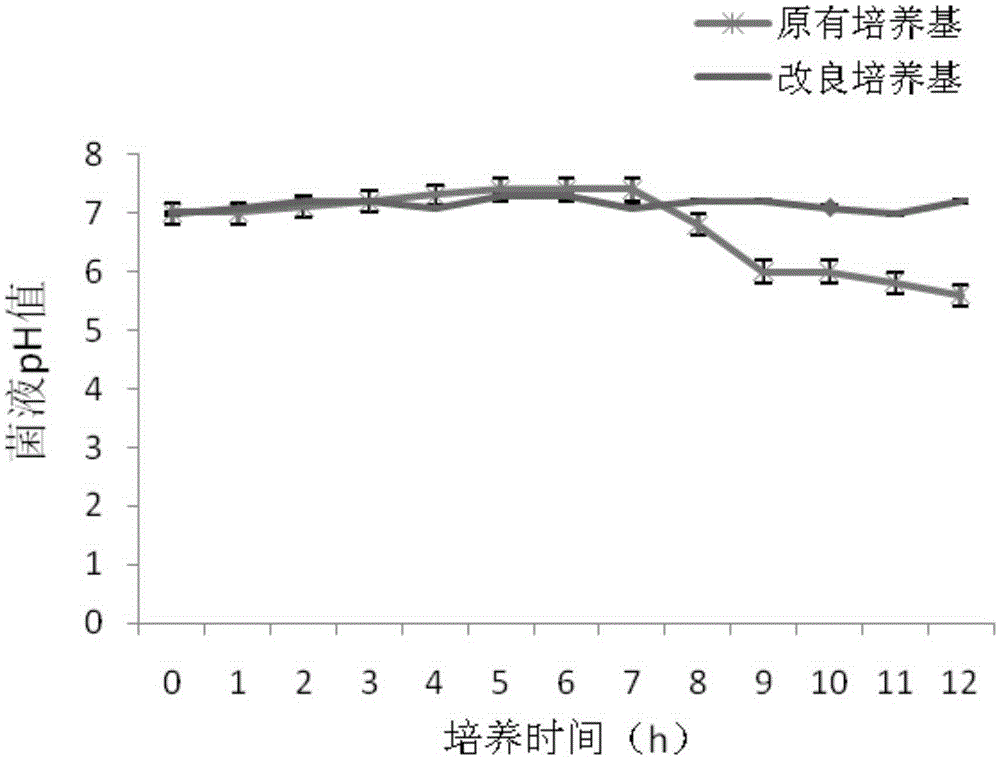
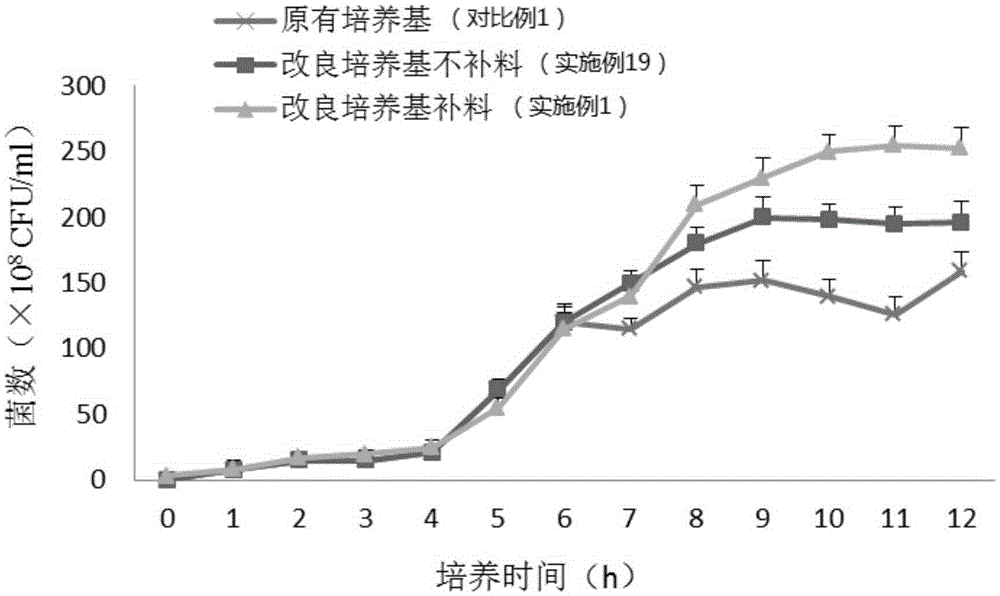
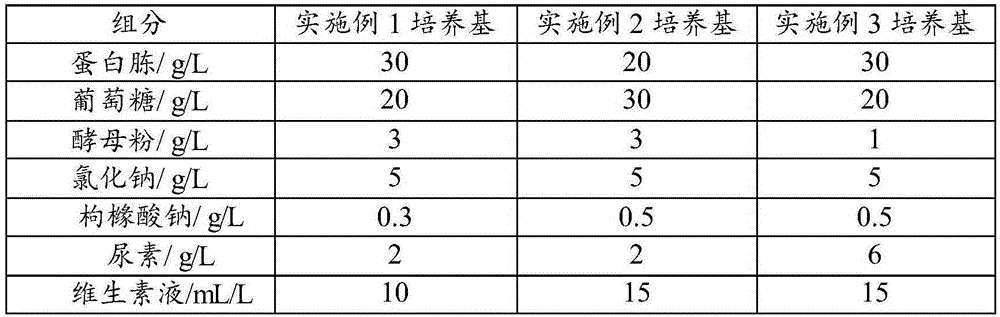
Pseudomonas aeruginosa fermentation medium, fermentation culture method thereof and vaccine preparation method
A fermentation medium and fermentation culture technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., can solve the problems of not fully utilizing the nutrient content of the medium, cumbersome process, and easy pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0178] Embodiment 1~3 fermentation medium and fermentation culture method
[0179] The fermentation medium composition in the present embodiment is as shown in the table below:
[0180] Table 1 Fermentation medium composition
[0181]
[0182]
[0183] The fermentation production process is as follows:
[0184] First-level seed preparation: Take 1 tube of DL15 strain and JL08 strain of freeze-dried strains, unseal each, dilute with sterilized saline, inoculate PYG agar medium respectively, culture at 37°C for 24 hours, select more than 5 typical colonies and mix with a small amount of PYG In the liquid medium, some PYG agar slant medium was inoculated again, and cultivated at 37°C for 24 hours as the primary seed. Store at 2-8°C, use for no more than 15 days, and pass for no more than 5 generations.
[0185] Secondary seed preparation: Inoculate several primary seeds into 5mL PYG liquid medium, shake at 37°C for 12 hours, then inoculate a larger amount of PYG liquid m...
Embodiment 4~6
[0192] Embodiment 4~6 fermentation culture medium and fermentation culture method
[0193] The fermentation medium composition in the present embodiment is as shown in the table below:
[0194] Table 3 Fermentation Medium Components
[0195] components
Example 4 culture medium
Example 5 culture medium
Example 6 culture medium
Peptone / g / L
10
20
30
Glucose / g / L
10
20
30
1
2
3
6
6
6
Sodium citrate / g / L
0.1
0.1
0.1
Urea / g / L
2
4
6
Vitamin solution / mL / L
10
15
20
Na 2 HPO 4 / g / L
1
1
1
K H 2 PO 4 / g / L
0.4
0.4
0.4
MgSO 4 / g / L
0.1
0.3
0.5
water
make up
make up
make up
[0196]The fermentation production process is as follows:
[0197] First-level seed preparation: Take 1 tube of DL15 strain and JL08 strain o...
Embodiment 7~9
[0205] Embodiment 7~9 fermentation culture medium and fermentation culture method
[0206] The fermentation medium composition in the present embodiment is as shown in the table below:
[0207] Table 5 Fermentation Medium Components
[0208] components
Example 7 culture medium
Example 8 culture medium
Example 9 culture medium
Peptone / g / L
10
20
30
Glucose / g / L
30
10
10
1
2
1
4
4
4
Sodium citrate / g / L
0.3
0.3
0.5
Urea / g / L
4
6
4
Vitamin solution / mL / L
15
20
20
Na 2 HPO 4 / g / L
5
5
5
K H 2 PO 4 / g / L
1.6
1.6
1.6
MgSO 4 / g / L
0.5
0.1
0.3
water
make up
make up
make up
[0209] The fermentation production process is as follows:
[0210] First-level seed preparation: Take 1 tube of DL15 strain and JL08 strain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com