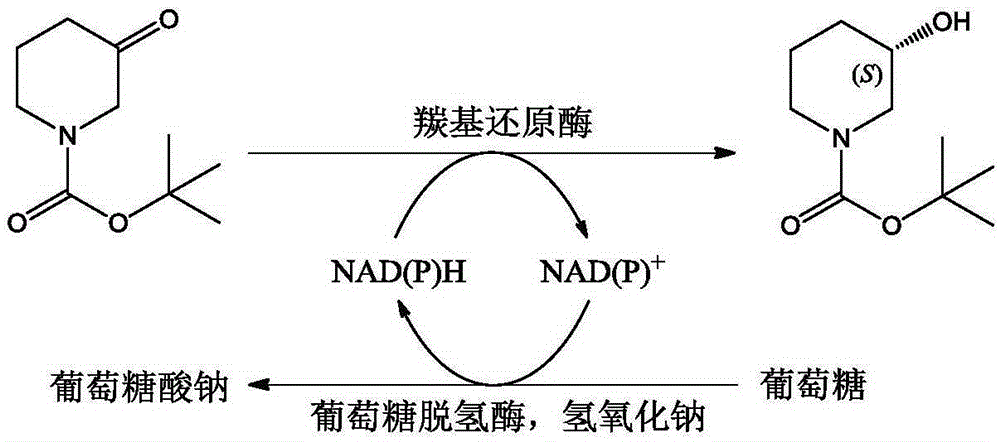
Method for preparing (S)-N-t-butyloxycarboryl-3-hydroxypiperidine
A carbonyl and oxidized coenzyme technology, which is applied in fermentation and other fields, can solve the problems of difficult catalyst acquisition, poor substrate tolerance, and low space-time yield, and achieve important industrial application value, mild reaction conditions, and high reaction efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Preparation of recombinant carbonyl reductase crude enzyme solution
[0033] The biocatalyst of the present invention can be prepared by conventional methods in the art.
[0034] Construction of recombinant bacteria with carbonyl reductase: The fragment containing the carbonyl reductase gene and the pET28a plasmid were double-digested with the same restriction endonuclease, ligated and the ligated product was transferred into a competent E.coliBL21(DE3) strain and screened to obtain Positive clones were inoculated into LB liquid medium containing kanamycin resistance (50 μg / mL) and activated overnight (37° C., 220 rpm) to prepare seed liquid. Transfer the seed solution to 100 mL of LB liquid medium containing kanamycin resistance (50 μg / mL) with 1% inoculum, and culture it with shaking at 37 ° C and 220 rpm until OD 600 When the temperature is between 0.6 and 0.8, add IPTG (0.5 mM) and continue culturing overnight at 20°C. The cells were collected by cent...
Embodiment 2
[0035] Embodiment 2 carbonyl reductase biocatalysis
[0036] A certain amount of substrate and 50 μL of methanol were added to a 10 mL reaction bottle, and then 0.1 mL of potassium phosphate buffer (1M, pH 7.0), 0.1 mL of recombinant carbonyl reductase crude enzyme solution (30 mg / mL, 6.27 U / mg ), 0.025mLNADP + Solution (stock solution concentration 40mM), 0.1mL glucose dehydrogenase crude enzyme solution (30mg / mL, 10U / mg), 0.3mL glucose syrup (stock solution concentration 100%, w / v), add water to 1mL, mix well Afterwards, the reaction was magnetically stirred at 30°C, and the pH of the reaction system was adjusted to 6.5-7.0 with 1M sodium hydroxide solution every 15 minutes. Carry out HPLC analysis after reaction finishes, and detection result is as follows:
[0037] serial number
Embodiment 3 100
[0038] Embodiment 3 100 milligram level preparation technology
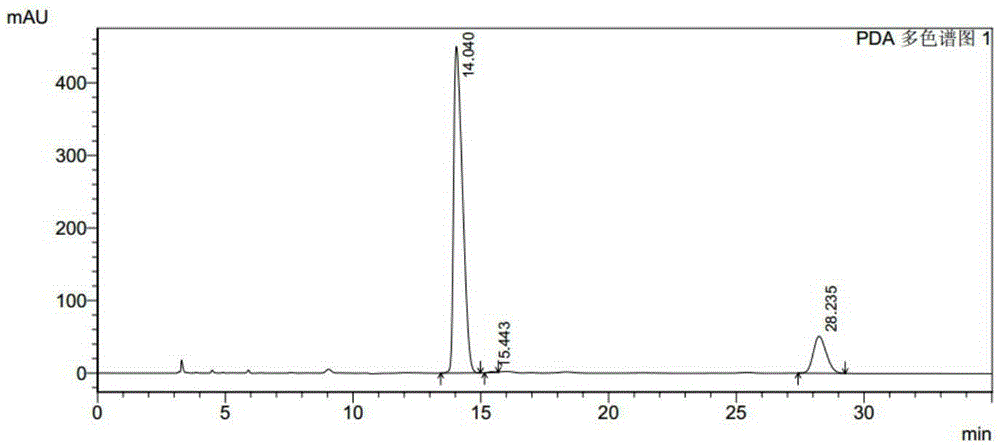
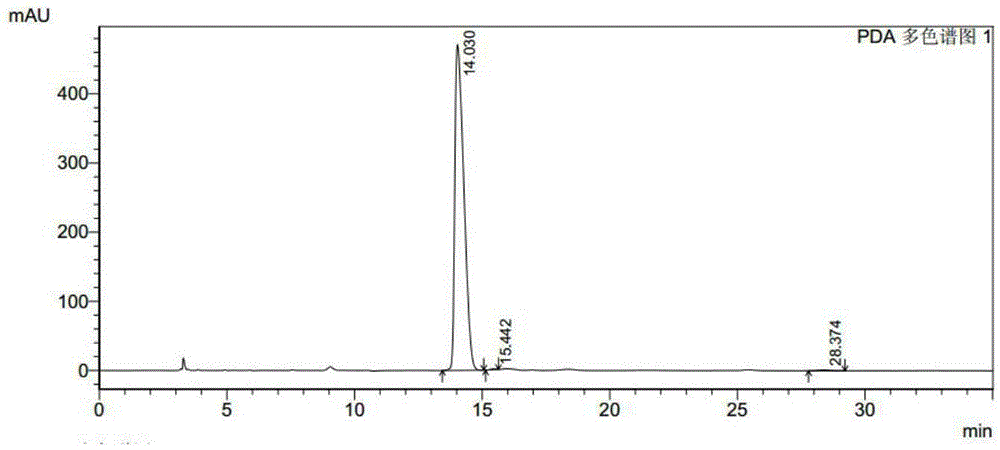
[0039] Add 150mg substrate and 50μL methanol to a 10mL reaction bottle, then add 0.1mL potassium phosphate buffer (1M, pH7.0), 0.1mL recombinant carbonyl reductase ChKRED03 crude enzyme solution (30mg / mL, 4.5U / mg ), 0.025mLNADP + Solution (stock solution concentration 40mM), 0.1mL glucose dehydrogenase crude enzyme solution (30mg / mL, 10U / mg), 0.18mL glucose syrup (stock solution concentration 100%, w / v), make up to 1mL with water, mix well Afterwards, the reaction was magnetically stirred at 30°C, and the pH of the reaction system was adjusted to 6.5-7.0 with 1M sodium hydroxide solution every 15 minutes. After reacting for 1h and 2.5h, samples were taken for HPLC analysis, and the spectra were shown in the attached instructions. figure 2 with image 3 . Instructions attached figure 2The retention time of 14.040min is (S)-N-tert-butoxycarbonyl-3-hydroxypiperidine, the retention time of 15.443 is the R conf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com