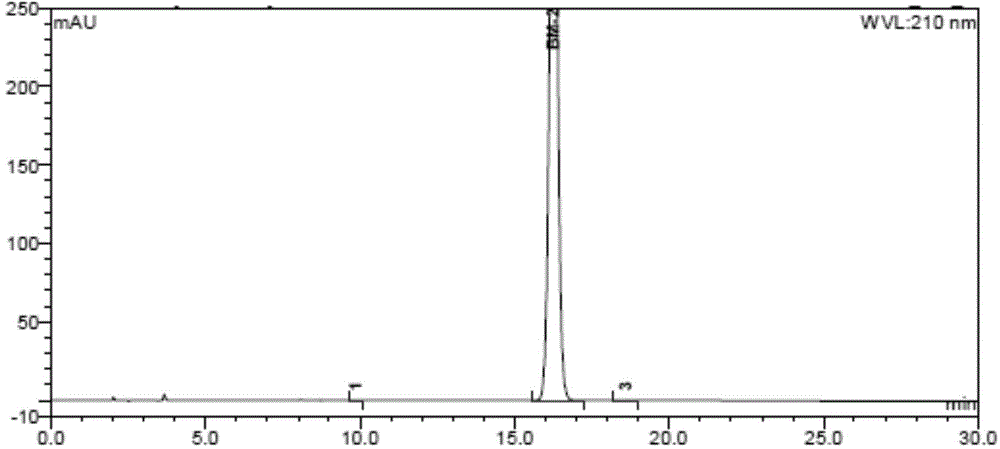
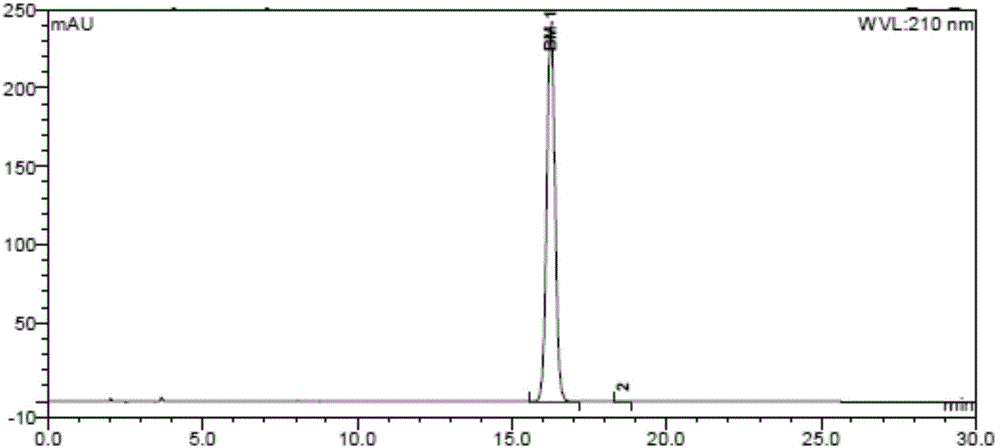
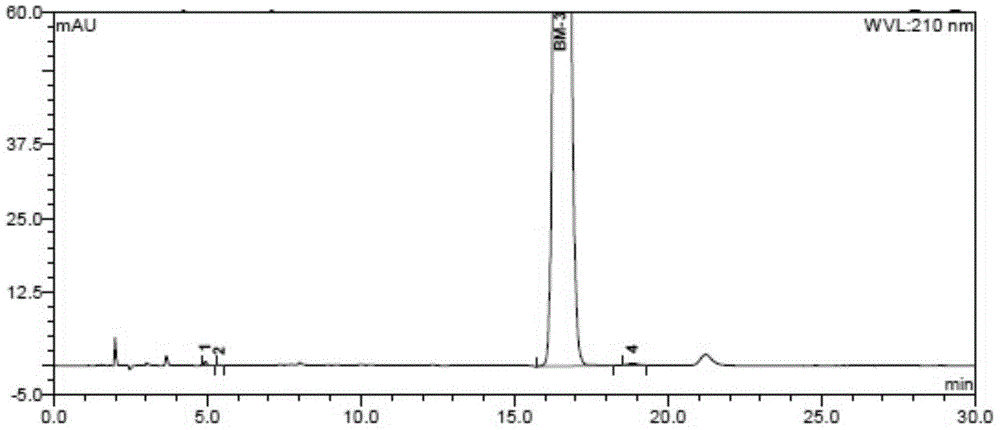
Preparation method of pomalidomide
A technology of pomalidomide and glutamine, which is applied in the field of preparing pomalidomide, can solve the problems of difficult industrialization, low product purity, and long reaction cycle, and achieve low production cost, good product quality, and short reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of formula IV compound
[0043] 19.32g of 3-nitrophthalic anhydride, 14.62g of D,L-isoglutamine, 8.92g of sodium acetate, and 100ml of acetic acid were stirred, refluxed, kept stirring for 7 hours, cooled to room temperature, filtered, washed with water, After drying, 29.86 g of gray solid was obtained, with a yield of 93.2%.
[0044] 1HNMR (500MHz, CDCl3) δ14.41(s, 1H), 11.48(s, 1H), 8.12(dd, J=7.5, 2.0Hz, 1H), 7.87(dd, J=7.5, 2.0Hz, 1H), 7.73(t, J=7.5Hz, 1H), 7.45(s, 1H), 4.51(dt, J=5.8, 1.1Hz, 1H), 3.19–3.10(m, 1H), 2.96(ddd, J=12.4, 9.0,7.6Hz,1H),2.08(dtd,J=12.4,8.9,5.8Hz,1H),1.63(ddt,J=12.4,7.7,1.4Hz,1H).
[0045] (2) preparation of formula VI compound
[0046] 10 g of the compound of formula IV and 30 ml of DMF were added to the reaction flask, and 5 g of thionyl chloride was added dropwise with stirring at -20°C. Insulation reaction 2-3 hours. The reaction solution was poured into rapidly stirred ice water, extracted with 2×50ml ethyl acetat...
Embodiment 2
[0052] (1) preparation of formula V compound
[0053] 19.32g of 3-nitrophthalic anhydride, 17.6g of D,L-dimethyl glutamate, 8.92g of sodium acetate, and 100ml of acetic acid were stirred, refluxed, kept stirring for 7 hours, cooled to room temperature and filtered. Washed with water and dried to obtain a gray solid, filtered and dried to obtain 34.16 g of the product with a yield of 97.6%.
[0054] 1 HNMR (500MHz, DMSO-d 6 )δ8.11(dd, J=7.4,1.9Hz,1H),8.03(dd,J=7.4,1.9Hz,1H),7.74(t,J=7.4Hz,1H),4.29(t,J=6.9 Hz,1H),3.64(d,J=10.2Hz,6H),2.77(ddd,J=12.3,5.2,1.8Hz,1H),2.55(td,J=12.1,4.5Hz,1H),2.46(dddd ,J=11.8,6.7,4.7,1.9Hz,1H),2.29(tdd,J=12.2,6.9,5.2Hz,1H).
[0055] (2) preparation of formula VI compound
[0056] Add 30 g of the compound of formula V, 100 ml of DMF, and 0.41 g of sodium amide into the reaction flask. 150°C heat preservation reaction for 5 hours. The reaction solution was concentrated under reduced pressure at 60°C, poured into rapidly stirred ice water, and a ...
Embodiment 3
[0063] (1) Preparation of formula IV compound
[0064] 19.66g of 3-nitrophthalic anhydride, 14.73g of D,L-isoglutamine, 3.68g of triethylamine, stirred in 100ml of DMF, reflux reaction, kept stirring for 9 hours, cooled to room temperature, filtered, washed with water, After drying, 23.89 g of gray solid was obtained, with a yield of 74.56%.
[0065] (2) preparation of formula VI compound
[0066] Add 10 g of the compound of formula IV and 50 ml of tetrahydrofuran into the reaction flask, and add 13.12 g of CDI in batches under stirring at 10°C. After the addition, the reaction was refluxed for 2 to 3 hours. After concentration, pour into rapidly stirred ice water, extract with 2×50ml ethyl acetate, and dry over anhydrous sodium sulfate. Sodium sulfate was removed by filtration, and the mother liquor was concentrated to dryness under reduced pressure. Stir in acetic acid at room temperature for one hour, filter, wash with water, and dry to obtain 4.17 g of the product with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com