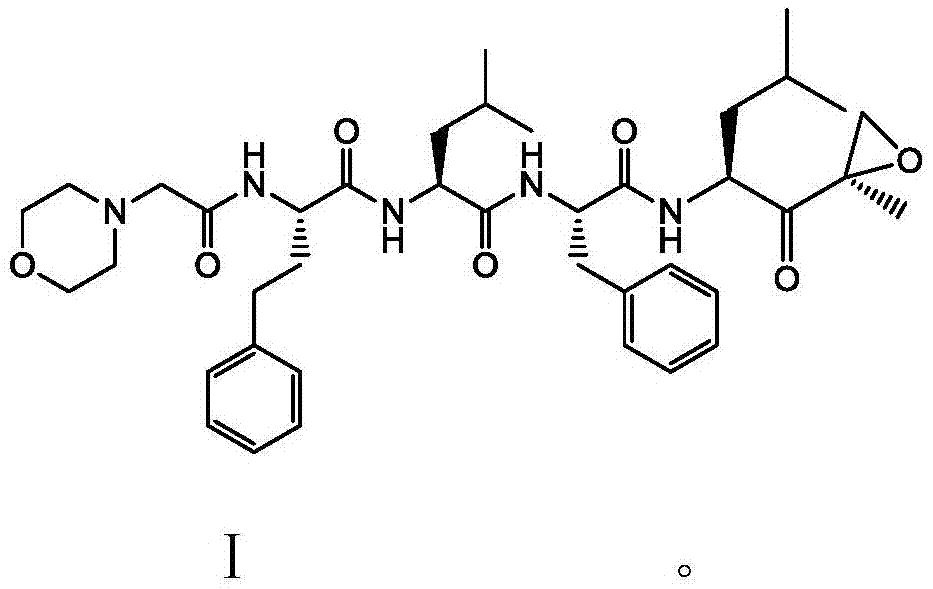
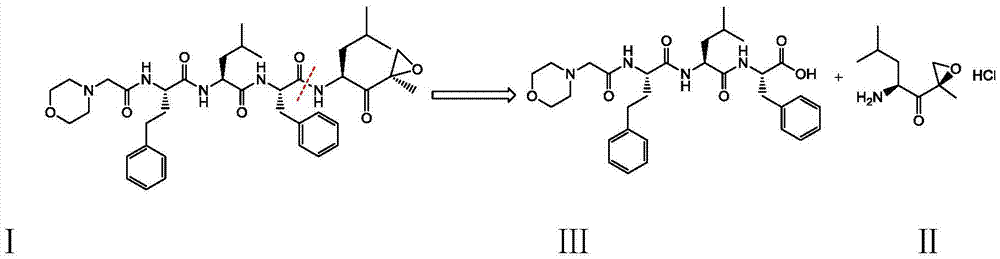
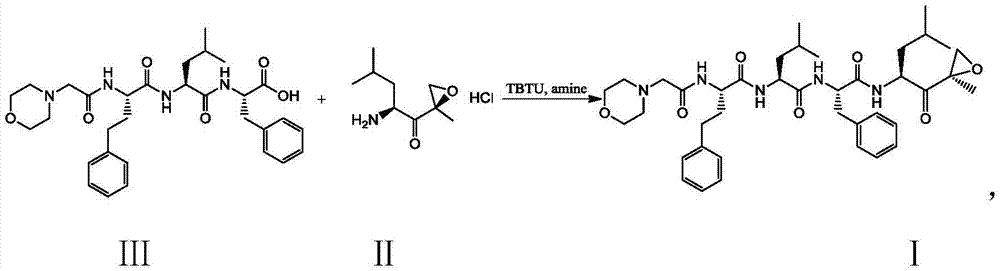
Preparation method of carfilzomib
A carfilzomib and compound technology, applied in the direction of peptides, etc., can solve the problems of difficult removal of by-products, harsh reaction conditions, high temperature control requirements, etc., and achieve the effects of easy removal, short reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Compound Ⅱ (10.4g, 50mmol), compound Ⅲ (29.7g, 52.5mmol) and TBTU (19.3g, 60mmol) were dissolved in 200ml of dichloromethane, placed in an ice-water bath, and then N,N-diisopropyl Dichloromethane solution of ethylamine (13.0g, 100mmol), remove the ice-water bath after the addition, stir at room temperature for 2h to obtain a reaction solution, add 2% sodium bicarbonate solution (500ml×2) to wash, and then wash with saturated saline (500ml×2), dried with anhydrous sodium sulfate (10g), concentrated under reduced pressure to obtain a viscous crude product, and then beaten with a mixture of n-hexane and ethyl acetate (volume ratio 5:1) in a certain volume ratio After 12 hours, it was filtered and dried to obtain 32.0 g of the target product carfilzomib as a white solid with a yield of 89.0%.
Embodiment 2
[0032] Compound II (10.4g, 50mmol), compound III (29.7g, 52.5mmol) and TBTU (19.3g, 60mmol) were dissolved in 200ml of dichloromethane, placed in an ice-water bath, and then triethylamine (10.1g, 100mmol) of dichloromethane solution, remove the ice-water bath after the addition, and stir at room temperature for 2h to obtain a reaction solution, add 2% sodium bicarbonate solution (500ml×2) to wash, and then wash with saturated brine (500ml×2), Dry over anhydrous sodium sulfate (10g), concentrate under reduced pressure to obtain a viscous crude product, then use a certain volume ratio of n-hexane and ethyl acetate (volume ratio: 5:1) to beat for 12 hours, filter, and dry to obtain The target product, carfilzomib, was 30.6 g of white solid, and the yield was 86.0%.
Embodiment 3
[0034] Take 32.0 g of the crude carfilzomib obtained above, heat it to 70-80°C with ethanol (128ml), cool down to below 0°C after dissolving, and filter after crystallization for 3 hours. The filter cake is rinsed with a small amount of ethanol. Dry in a blast oven at 50°C to obtain 26.9 g, with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com