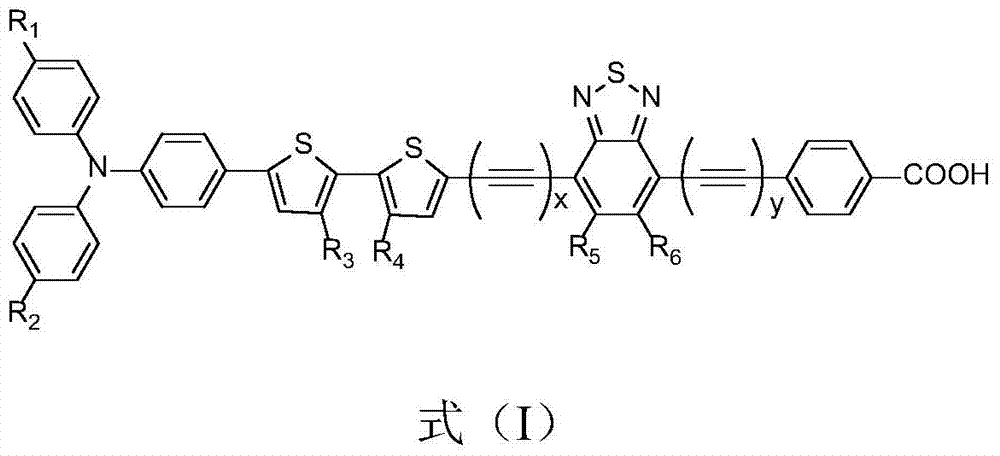
An organic dye, its preparation method and a sensitized solar cell containing the organic dye
A technology of organic dyes and solar cells, applied in the field of dye-sensitized solar cells and sensitized solar cells, can solve the problems of dye-sensitized solar cells that cannot be industrialized on a large scale, and the photothermal stability of devices is not good, and achieve industrial production Bright prospects, low cost, and good photothermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of organic dye provided by the invention comprises the following steps:
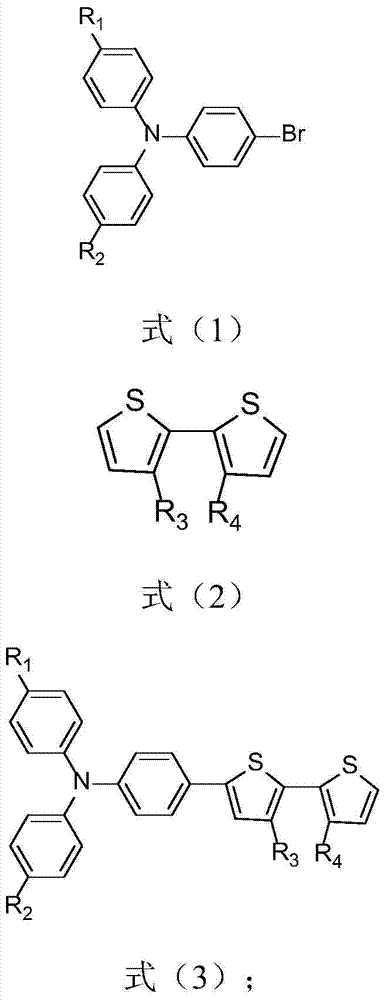
[0040] Step 1, have the compound of formula (1) structural formula in Pd(OAc) 2 , PCy 3 ·HBF 4 , PivOH and K 2 CO 3 Under the action, the compound with the structural formula of the formula (3) is obtained through the coupling reaction of the compound of the structural formula of the formula (2) through the activation of the C-H bond;
[0041]
[0042] Step 2-1, have the compound of formula (3) structural formula in Pd(OAc) 2 , PCy 3 ·HBF 4 , PivOH and K 2 CO 3 Under the action, the dye precursor butyl ester compound is obtained through the C-H bond activation and the compound having the formula (4) for coupling reaction;
[0043]
[0044]Or step 2-2, the compound with formula (3) structural formula carries out bromination with NBS reagent, and the bromination product obtained is in Pd(PPh 3 ) 2 Cl 2 , triphenylphosphine, cuprous iodide, and diisopropylamin...
Embodiment 1
[0053]
[0054] Compound 2 was synthesized according to references (M. Zhang, Y. Wang, M. Xu, W. Ma, R. Li, P. Wang, Energy Environ. Sci., 2013, 6, 2944). In the present invention, the sources of other raw material compounds, solvents, and catalysts in the process of preparing organic dyes are not particularly limited, and can be commercially available or prepared according to methods known in the art.
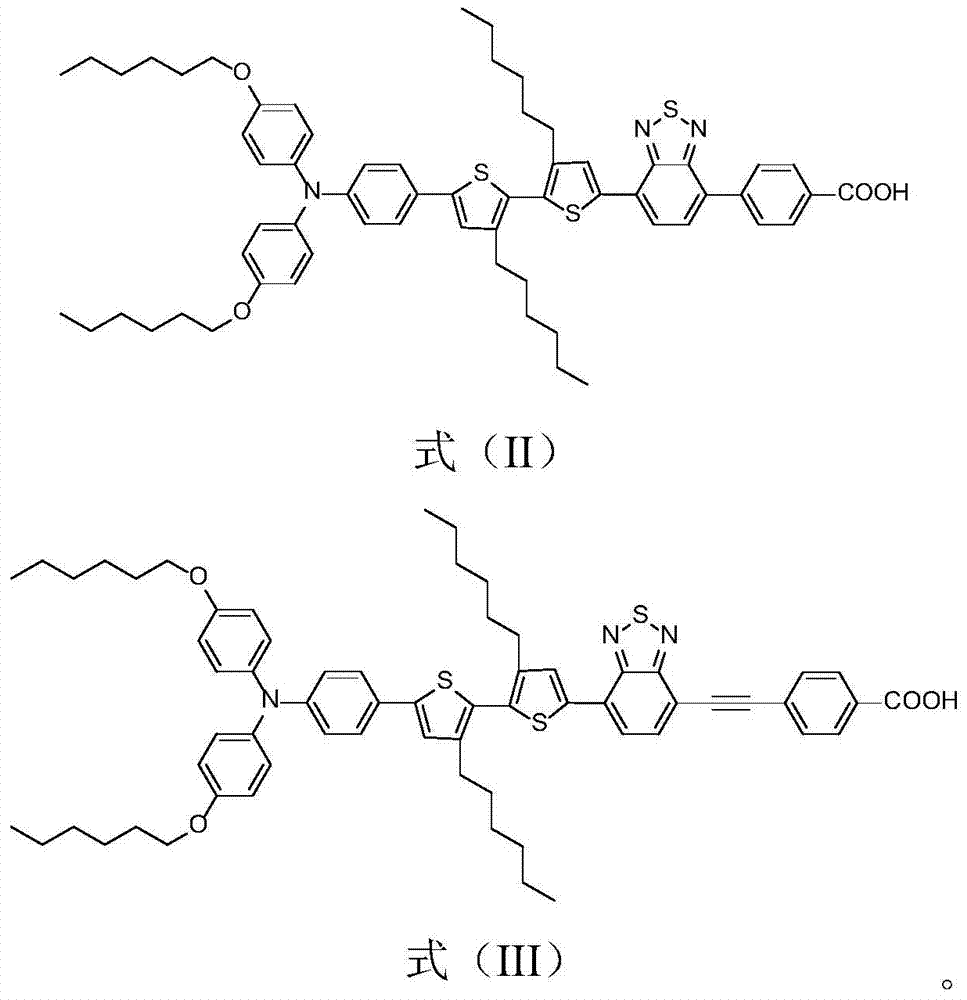
[0055] Synthesis of compound shown in formula (II):
[0056] In a three-neck round bottom flask, 2.70 g of compound 1 was dissolved in 40 ml of toluene, and 1.62 g of compound 2, 45 mg of Pd(OAc) were added to the reaction system under the protection of argon. 2 , 151 mg PCy 3 ·HBF 4 , 104 mg PivOH and 705 mg K 2 CO 3 , heated to reflux, and reacted overnight with stirring.
[0057] After the reaction was finished, the reaction system was cooled to room temperature, 40 ml of water was added, the resulting mixed solution was extracted three times with chloroform, the or...
Embodiment 2
[0063]
[0064] Compound 3 was synthesized according to literature (Z. Yao, H. Wu, Y. Li, J. Wang, J. Zhang, M. Zhang, Y. Guo and P. Wang, Energy Environ. Sci., 2015, 8, 3192). In the present invention, the sources of other raw material compounds, solvents, and catalysts in the process of preparing organic dyes are not particularly limited, and can be commercially available or prepared according to methods known in the art.
[0065] Synthesis of target product III:
[0066] In a three-neck round bottom flask, 2.70 g of compound 1 was dissolved in 40 ml of toluene, and 1.72 g of compound 3, 45 mg of Pd(OAc) were added to the reaction system under the protection of argon. 2 , 151 mg PCy 3 ·HBF 4 , 104 mg PivOH and 705 mg K 2 CO 3 , heated to reflux, and reacted overnight with stirring.
[0067] After the reaction was finished, the reaction system was cooled to room temperature, 40 ml of water was added, the resulting mixed solution was extracted three times with chlorofo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com