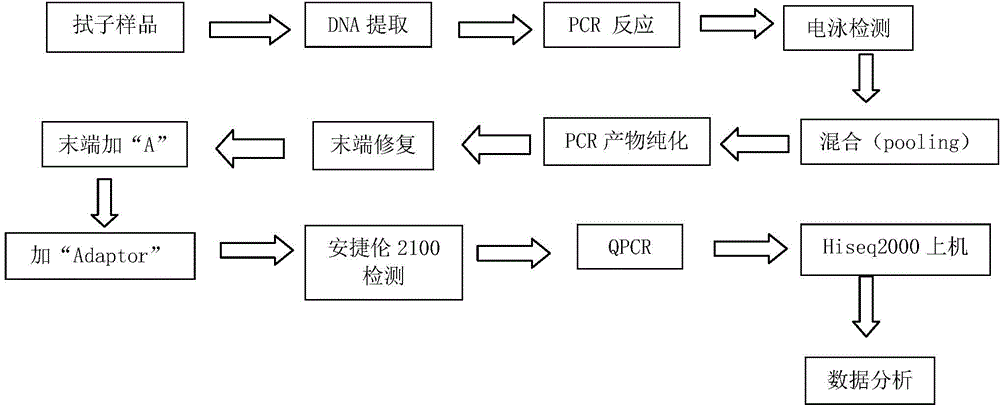
Kit and application thereof in genital tract pathogen proliferation testing
A kit and pathogen technology, applied in the field of microbial detection, can solve problems such as clinical diagnosis and treatment obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0030] According to a specific embodiment of the present invention, the detection method also includes:
[0031] (5) Determine whether the host is infected with certain pathogen(s).
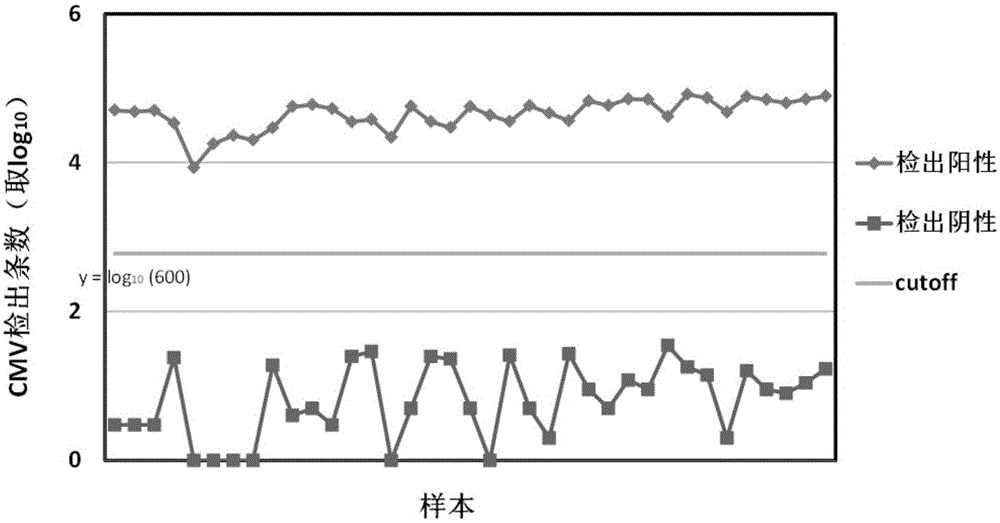
[0032] Using the comparison results in (4), calculate the abundance of various pathogens contained in the sample to be tested, and compare the abundance of each pathogen with the non-pathogenic abundance of this pathogen. If there is a significant difference between the two, then Confirm that the host is infected with the pathogen. The abundance here reflects the amount of the pathogen in that sample. In a specific embodiment of the present invention, the determination of the non-pathogenic abundance of a certain pathogen, that is, the determination of the threshold, the threshold can be a specific value or a range of values or even a formula, including: from multiple negative blood / Obtain multiple non-pathogenic abundances of a certain pathogen in plasma / serum samples, which can be obtain...
Embodiment
[0038] Patient urine / vaginal and cervical secretion samples (swab samples) came from Inner Mongolia Maternal and Child Health Hospital. IlluminaHiseq high-throughput sequencing technology was used to test whether the patient samples contained Ureaplasma urealyticum (UU), Neisseria gonorrhoeae (NG), group B streptococcus (GBS), Candida albicans (CA), vaginal Gardner bacteria (GV), Trichomonas vaginalis (TV), herpes simplex virus (HSV), Treponema pallidum (TP), Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Mycoplasma hominis (MH), Haemophilus dukei (HD) and / or cytomegalovirus (CMV) for detection, the operation steps are as follows:
[0039] 1. Primer Design
[0040] Firstly, according to the genome sequences of UU, NG, GBS, CA, GV, TV, HSV, TP, CT, MG, MH, HD, and CMV, PCR primer pairs were designed respectively, and multiple tests were screened to obtain the primers that can be used in the same PCR reaction system. Primer sequences for multiplex amplification.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com