Dissolved air flotation, antisolvent crystallisation and membrane separation for separating buoyant materials and salts from water
A technology of buoyancy materials and hydrophobic parts, which is applied in evaporation separation crystallization, semi-permeable membrane separation, flotation, etc., and can solve problems such as large diameter of packed tower, easy blockage of equipment, insolubility of silver salt, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0489] The following examples further illustrate the principles of the above-described aspects of the invention.
[0490] 1. Regarding the separation of elements such as strontium from liquids
[0491] a. Background
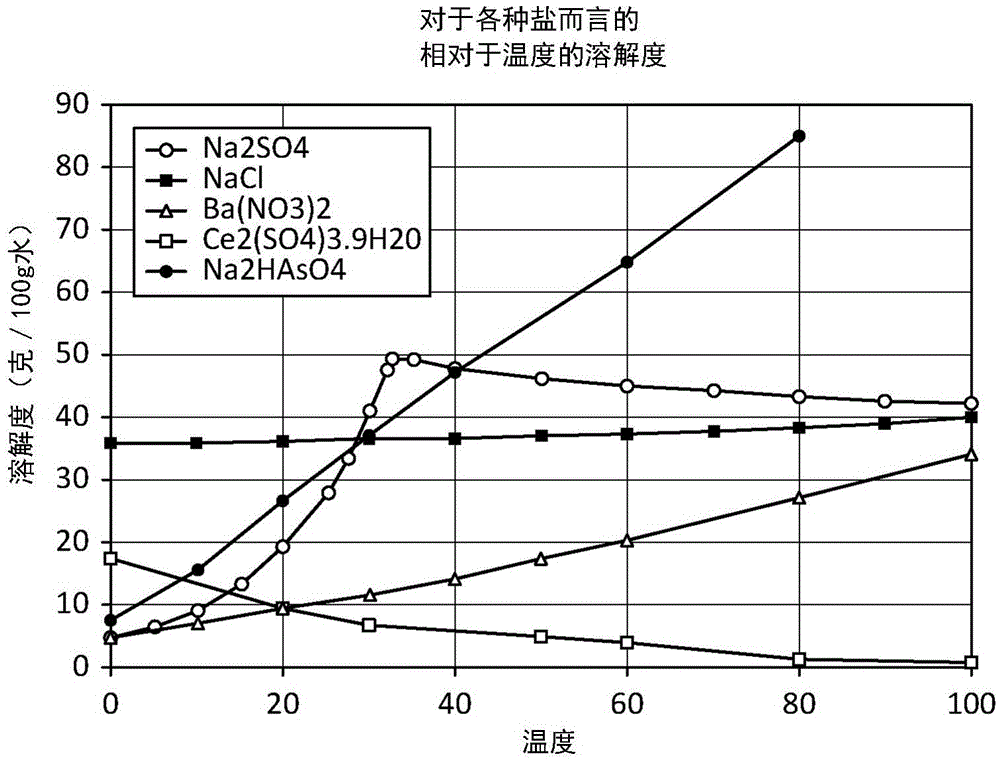
[0492] As noted above, typically produced / brackish water may contain strontium along with calcium and other inorganic contaminants. Normally, calcium is present in much higher concentrations than strontium in the feed water, where calcium ++ / strontium ++ The ratio is in the range of 10-50, and since the two contaminants are very similar in the aqueous solubility of their salts, it is difficult to preferentially separate strontium.
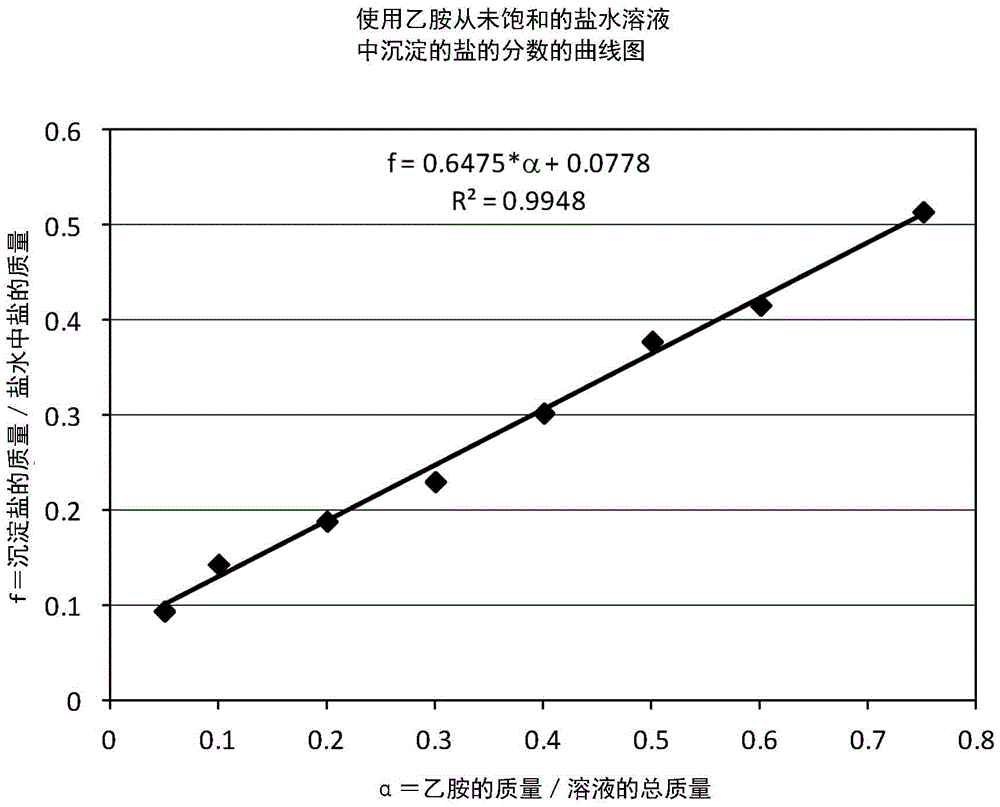
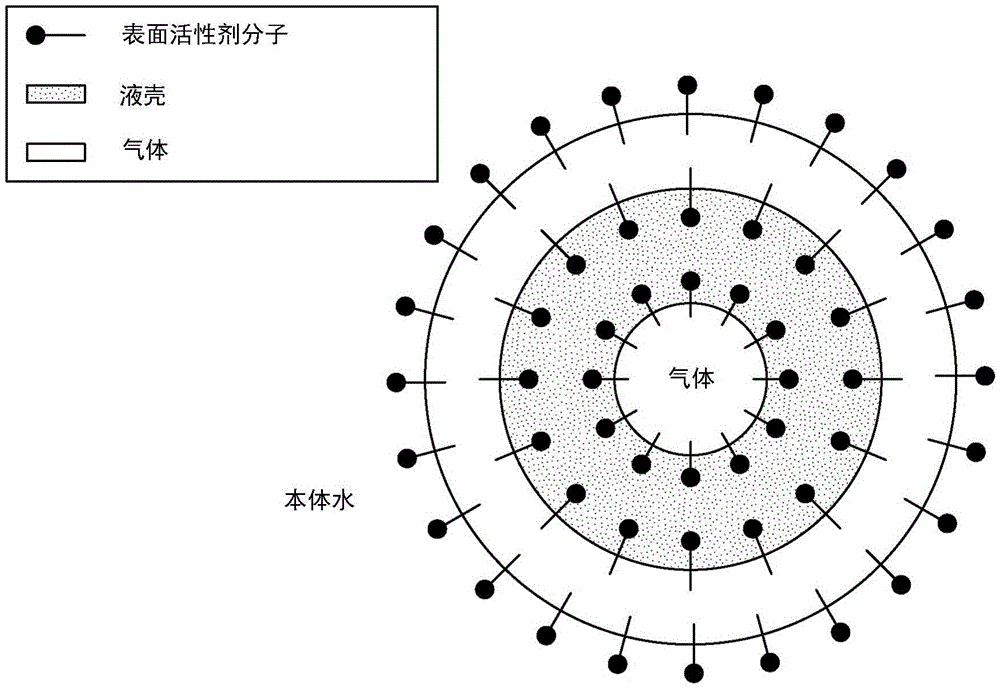
[0493] One aspect of the present invention is the initial separation of strontium from produced / brackish water with little or no calcium precipitation, even though the concentration of calcium is much higher than that of strontium. The basic idea, as described above, is to preferentially precipitate strontium out of water by prem...
example 1
[0501] In this example, 500 ml of produced water containing 442 mg / L strontium chloride and 6200 mg / L calcium chloride (given Ca ++ / Sr ++ The weight ratio is 14.0 and the molar ratio is 20.0). Keep the temperature at 25°C and add a small amount of sodium sulfate (Na 2 SO 4 ), and mixed with 500 ml of water in a constantly stirring beaker. The total amount of sodium sulfate added was 280 mg. About 10 minutes after each incremental addition, the Sr ++ The concentration and weight percent removal of strontium was calculated by the difference from the initial amount present in the water. Table 5 gives the % of sodium sulfate used to precipitate strontium.
[0502] table 5
[0503] sample
example 2
[0504] C. Example 2: Selective Precipitation of Strontium
[0505] The strontium-containing water was first reacted with sulfuric acid based on the incoming strontium concentration, wherein a 40% excess of sulfuric acid was added to complete the formation of strontium sulfate in the water. The pH of the water is below 3.0.
[0506] In this example 1 liter of produced water containing 442 mg / L strontium chloride, 6200 mg / L calcium chloride and 30 mg / L barium chloride was used (given calcium ++ / strontium ++ The weight ratio is 14.0 and the molar ratio is 20.0). The temperature was maintained at 25°C.
[0507] The chemical precipitation reaction is as follows:
[0508] SrCl 2 +H 2 SO 4 →SrSO 4 +2HCl
[0509] BaCl 2 +H 2 SO 4 → BaSO 4 +2HCl
[0510] After the reaction was complete, which took about 1 hour, the pH was raised to a range between 3.5 and 4.0, which caused strontium sulfate and barium sulfate to precipitate out, while any calcium sulfate formed remaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com