Method for efficiently preparing quinoline derivative from titanocene dichloride in cooperation with with sulfo-benzoic acid in water phase
A technology of sulfobenzoic acid and titanocene dichloride, applied in the direction of organic chemistry, etc., to achieve the effect of mild reaction conditions, short reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
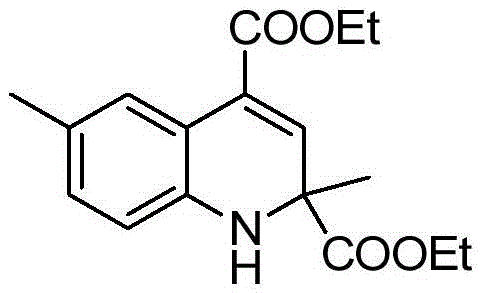
[0012] Taking the preparation of the compound diethyl-2,6-dimethyl-1,2-dihydroquinoline-2,4-dicarboxylic acid of the following formula as an example, the raw materials used and the preparation method are as follows:
[0013]
[0014] Add 0.0125g (0.05mmol) titanocene dichloride, 38μL (0.10mmol) 4-sulfophthalic acid, 0.1072g (1mmol) 4-methylaniline, and 329μL (3mmol) pyruvic acid to a 10mL Shrek tube Ethyl acetate and 1 mL of distilled water were stirred for 6 hours at 60°C. The reaction was stopped, and 10-15 mL of ethyl acetate was added for extraction. After the ethyl acetate was removed by rotary evaporation of the organic layer, it was separated and purified by silica gel column (eluent was ethyl acetate The volume ratio with petroleum ether is 1:10) to obtain diethyl-2,6-dimethyl-1,2-dihydroquinoline-2,4-dicarboxylic acid with a yield of 90% , The spectral data of the product is as follows:
[0015] 1 HNMR(400MHz, CDCl 3 )δ: 7.61(s, 1H), 6.88(d, J=7.5Hz, 1H), 6.65(s, 1H), 6.5...
Embodiment 2
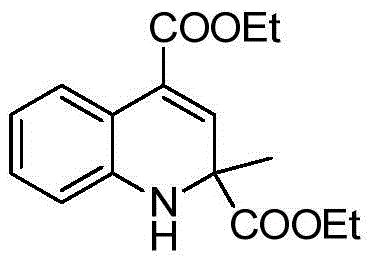
[0018] Taking the preparation of the compound diethyl-2-methyl-1,2-dihydroquinoline-2,4-dicarboxylic acid of the following formula as an example, the raw materials used and the preparation method are as follows:
[0019]
[0020] In Example 1, the 4-methylaniline used was replaced with an equimolar amount of aniline, and the other steps were the same as in Example 1, to obtain diethyl-2-methyl-1,2-dihydroquinoline-2, The yield of 4-dicarboxylic acid is 89%. The spectrum data of the product is as follows:
[0021] 1 HNMR(400MHz, CDCl 3 )δ: 7.71 (dd, J = 7.9, 1.0 Hz, 1H), 6.99 (dd, J = 7.7, 1.2 Hz, 1H), 6.65 (d, J = 0.6 Hz, 1H), 6.60-6.51 (m, 2H) ), 4.42(s, 1H), 4.26(dd, J=7.1, 1.3Hz, 2H), 4.18-4.01(m, 2H), 1.47(s, 3H), 1.30(t, J=7.1Hz, 3H) , 1.19 (t, J=7.1 Hz, 3H).
[0022] 13 CNMR(101MHz, CDCl 3 )δ: 172.87, 164.84, 141.58, 131.46, 128.55, 127.51, 125.49, 117.55, 115.55, 113.18, 60.77, 59.98, 57.49, 26.40, 13.23, 13.10.
Embodiment 3
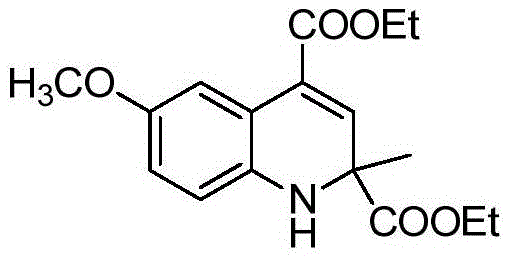
[0024] Taking the preparation of the compound diethyl-6-methoxy-2-methyl-1,2-dihydroquinoline-2,4-dicarboxylic acid of the following formula as an example, the raw materials used and the preparation method thereof are as follows:
[0025]
[0026] In Example 1, the 4-methylaniline used was replaced with an equimolar amount of 4-methoxyaniline, and the other steps were the same as in Example 1, to obtain diethyl-6-methoxy-2-methyl- The yield of 1,2-dihydroquinoline-2,4-dicarboxylic acid is 92%. The spectral data of the product is as follows:
[0027] 1 HNMR(400MHz, CDCl 3 )δ: 7.49 (d, J=2.8Hz, 1H), 6.74 (s, 1H), 6.70 (dd, J=8.6, 2.8Hz, 1H), 6.58 (d, J=8.6Hz, 1H), 4.38- 4.27 (m, 3H), 4.23-4.11 (m, 2H), 3.74 (s, 3H), 1.52 (s, 3H), 1.37 (s, 3H), 1.24 (s, 3H).
[0028] 13 CNMR(101MHz, CDCl 3 )δ: 175.46, 167.34, 142.05, 135.55, 134.09, 130.46, 130.00, 127.11, 118.00, 115.65, 63.08, 62.32, 59.93, 29.68, 28.58, 17.25, 15.66.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com