Method for producing zinc sheets and recycling ammonium chloride from indium contain wastewater
A technology of ammonium chloride and zinc flakes, applied in the direction of ammonium chloride, ammonium halide, improvement of process efficiency, etc., can solve the problems of small market demand of products, no economic benefits, large environmental pollution, etc., and achieve smooth market sales channels , The product has high added value and good economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
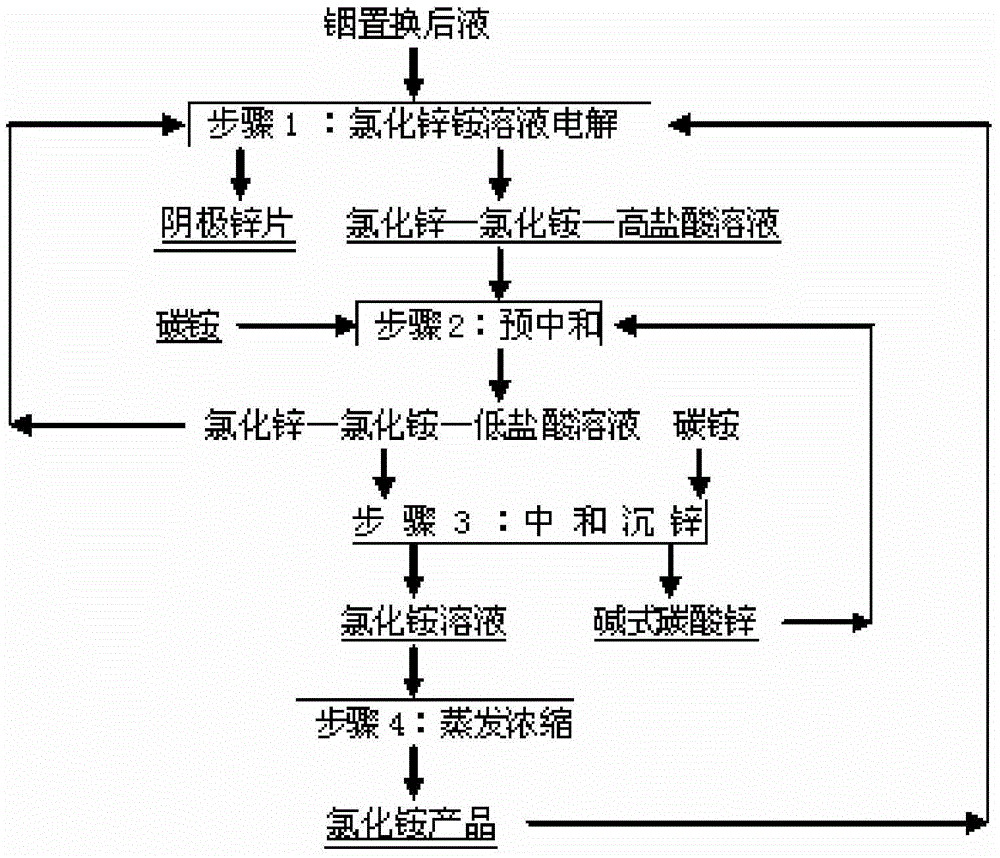
[0041] This embodiment is the first example of the method for producing zinc flakes and reclaiming ammonium chloride from the liquid after indium replacement described in the present invention, including the following steps:
[0042] (1), electrolysis of ammonium zinc chloride solution: will contain ZnCl 2 300g / L indium replacement liquid 10m 3 , mixed with ammonium chloride solid 790kg, prepared to contain ZnCl 2 300g / L, NH 4 Cl79g / L mixed solution 10m 3 , press the prepared mixed solution by 0.5m 3 The flow rate of / h is continuously and evenly added to the ZnCl-containing 2 90g / L, NH 4 In 20 plastic electrolyzers of Cl24g / L, HCl91g / L, the ZnCl-containing ZnCl that step (2) returns 2 120g / L, NH 4 Cl203g / L, HCl12g / L mixed solution 10m 3 by 0.5m 3 The flow rate per hour is continuously and evenly added to the 20 plastic electrolytic cells. The inner dimensions of each electrolytic cell are 1.5m long, 0.7m wide, and 1.0m high. Each electrolyzer is equipped with 11 grap...
no. 2 example
[0047] This embodiment is a second example of a method for producing zinc flakes and recovering ammonium chloride from liquid after indium replacement described in the present invention, comprising the following steps:
[0048] (1), electrolysis of ammonium zinc chloride solution: will contain ZnCl 2 400g / L indium replacement liquid 9m 3 , mixed with 950kg of ammonium chloride solid, prepared to contain ZnCl 2 400g / L, NH 4 Cl105g / L mixed solution 9m 3 , press the prepared mixed solution by 0.5m 3 The flow rate of / h is continuously and evenly added to the ZnCl-containing 2 120g / L, NH 4 In 18 plastic electrolyzers of Cl32g / L, HCl121g / L, start from the second circulation, the ZnCl that step (2) returns returns 2 160g / L, NH 4 9m of mixed solution of Cl271g / L and HCl16g / L 3 by 0.5m 3 The flow rate per hour is continuously and evenly added to the 18 plastic electrolytic cells. The inner dimensions of each electrolytic cell are 1.5m long, 0.7m wide, and 1.0m high. Each elec...
no. 3 example
[0053] This embodiment is the third example of the method for producing zinc flakes and recovering ammonium chloride from the liquid after indium replacement described in the present invention, including the following steps:
[0054] (1), electrolysis of ammonium zinc chloride solution: will contain ZnCl 2 500g / L indium replacement liquid 12m 3 , mixed with 1580kg of ammonium chloride solid, prepared to contain ZnCl 2 500g / L, NH 4 Cl132g / L mixed solution 12m 3 , press the prepared mixed solution by 0.6m 3 The flow rate of / h is continuously and evenly added to the ZnCl-containing 2 150g / L, NH 4 In 24 plastic electrolyzers of Cl40g / L, HCl152g / L, from the second circulation, the ZnCl-containing ZnCl returned by step (2) 2 200g / L, NH 4 Cl340g / L, HCl20g / L mixed solution 12m 3 by 0.6m 3 The flow rate per hour is continuously and evenly added to the 24 plastic electrolytic cells. The inner dimensions of each electrolytic cell are 1.5m long, 0.7m wide, and 1.0m high. Each el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com