Aliphatic nitrile catalytic oxidation synthesis method
A technology of catalytic oxidation and synthesis method, applied in the field of chemistry, can solve the problems of unsuitable fatty aldehyde, strict control requirements, unavoidable metal waste, etc., and achieve the effects of reducing environmental cost, easy and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
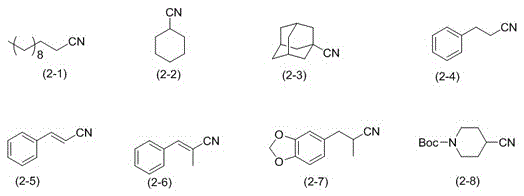
[0025] Example 1: Preparation of n-dodecanonitrile (formula (2-1))
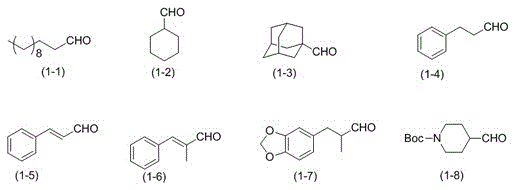
[0026] In a 100ml flask, add 50mL acetonitrile, 10mmolHMDS, 0.4mmol TEMPO, 0.4mmol KPF 6 And 0.6mmol of TBN, replace the air in the bottle with oxygen, seal the mouth of the bottle with a rubber stopper, insert an oxygen balloon, put the reaction bottle into a pre-heated water bath and heat to 30℃, slowly add 4mmol of dodecaldehyde (formula (1-1)), react for 8h. Sodium thiosulfate solution was added to the reaction solution and stirred, and then extracted with ether, the organic layer was separated, the solvent was distilled off under reduced pressure, and column chromatography was performed. The mixture of ethyl acetate / petroleum ether volume ratio 1:200 was used as Eluent, collect the eluent containing the target compound, evaporate the solvent to obtain dodeconitrile, the separation yield is 75%.
Embodiment 2
[0027] Example 2: Preparation of n-dodecanonitrile (formula (2-1))
[0028] The reaction steps are the same as in Example 1, except that acetonitrile is changed to N,N'-dimethylformamide and reacted for 20 hours. The isolated yield of dodeconitrile is 46%.
Embodiment 3
[0029] Example 3: Preparation of n-dodecanonitrile (formula (2-1))
[0030] The reaction steps are the same as in Example 1, except that acetonitrile is changed to dimethyl sulfoxide and reacted for 20 hours. The separation yield of dodeconitrile is 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com