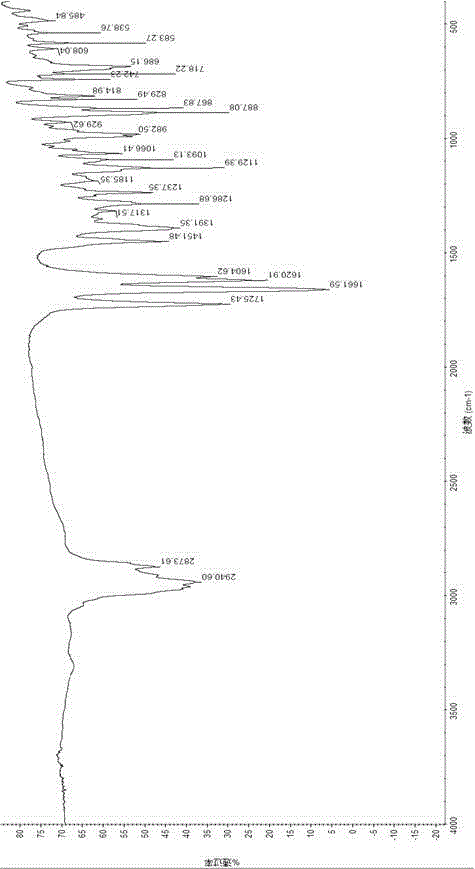
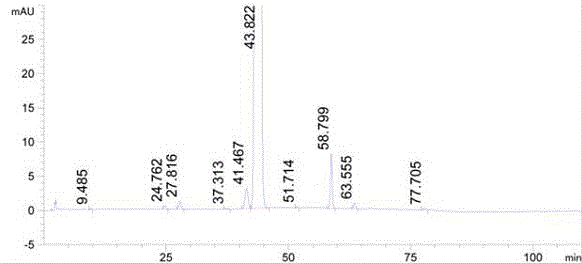
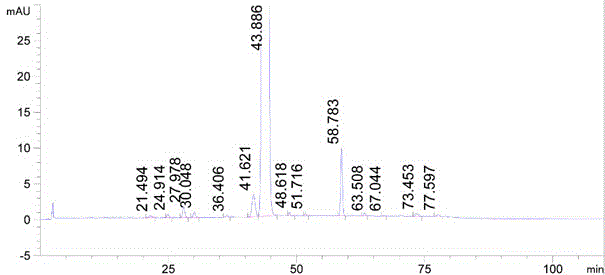
Method for synthesizing mometasone furoate
A technology of mometasone furoate and a compound is applied in the field of drug synthesis and achieves the effects of simple process, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of compound 1
[0029] Add 50 g of 9β, 11β-epoxy-17α, 21-dihydroxy-16α-methyl-1,4-pregna (steroid)diene-3,2-dione and 300 ml of pyridine into the reaction kettle. When the temperature of the reaction solution is lowered to -5~5°C, add 30g of p-toluenesulfonyl chloride into the reaction kettle, control the temperature at -5~5°C and react for 9h; pour the reaction solution into 3000ml of water, stir to precipitate solid, and discard the water layer , 380ml of dichloromethane was added to the residue, stirred for 10 minutes, separated, and the aqueous layer was extracted twice with 380ml of dichloromethane. The organic phase was adjusted to pH=6~7 with 1N hydrochloric acid, and the liquid was separated, and the organic phase was adjusted to pH=7~8 with saturated sodium bicarbonate solution; the organic phase was washed with 400ml of saturated saline; when the organic phase was concentrated to dryness, 150ml of dichloromethane was added Methane, 250ml of is...
Embodiment 2
[0037] (1) Preparation of compound 1
[0038]Add 50 g of 9β, 11β-epoxy-17α, 21-dihydroxy-16α-methyl-1,4-pregna (steroid)diene-3,2-dione and 300 ml of pyridine into the reaction kettle. When the temperature of the reaction solution is reduced to -5~5°C, add 15g of methanesulfonyl chloride into the reaction kettle, control the temperature at -5~5°C for 9 hours, and TLC detects that the reaction is complete; pour the reaction solution into 3000ml of water, and stir to precipitate a solid; Discard the water layer, add 380ml of dichloromethane to the residue, stir for 10 minutes, separate the layers, extract the water layer twice with 380ml of dichloromethane; Saturated sodium bicarbonate solution to adjust the pH=7~8; the organic phase was washed with 400ml of saturated saline; the organic phase was concentrated to dryness, and 150ml of dichloromethane was added, and 250ml of isopropyl ether was added dropwise at room temperature, and continued to stir for 3h, filtered, and dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com