Preparation of p-tolyl/4-chlorophenyl isocyanate-modified cationic cyclodextrin chiral resolution material through click chemistry and application of chiral resolution material
A technology of chlorophenylisocyanate and toluenesulfonyl cyclodextrin, which is applied in separation methods, solid adsorbent liquid separation, and other chemical processes, can solve the difficulties in the development of bonded arms and the unstable groups of functional bonded arms , Cyclodextrin falling off and other problems, to achieve the effect of improving chiral recognition ability, increasing separation throughput, and reducing the difficulty of immobilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
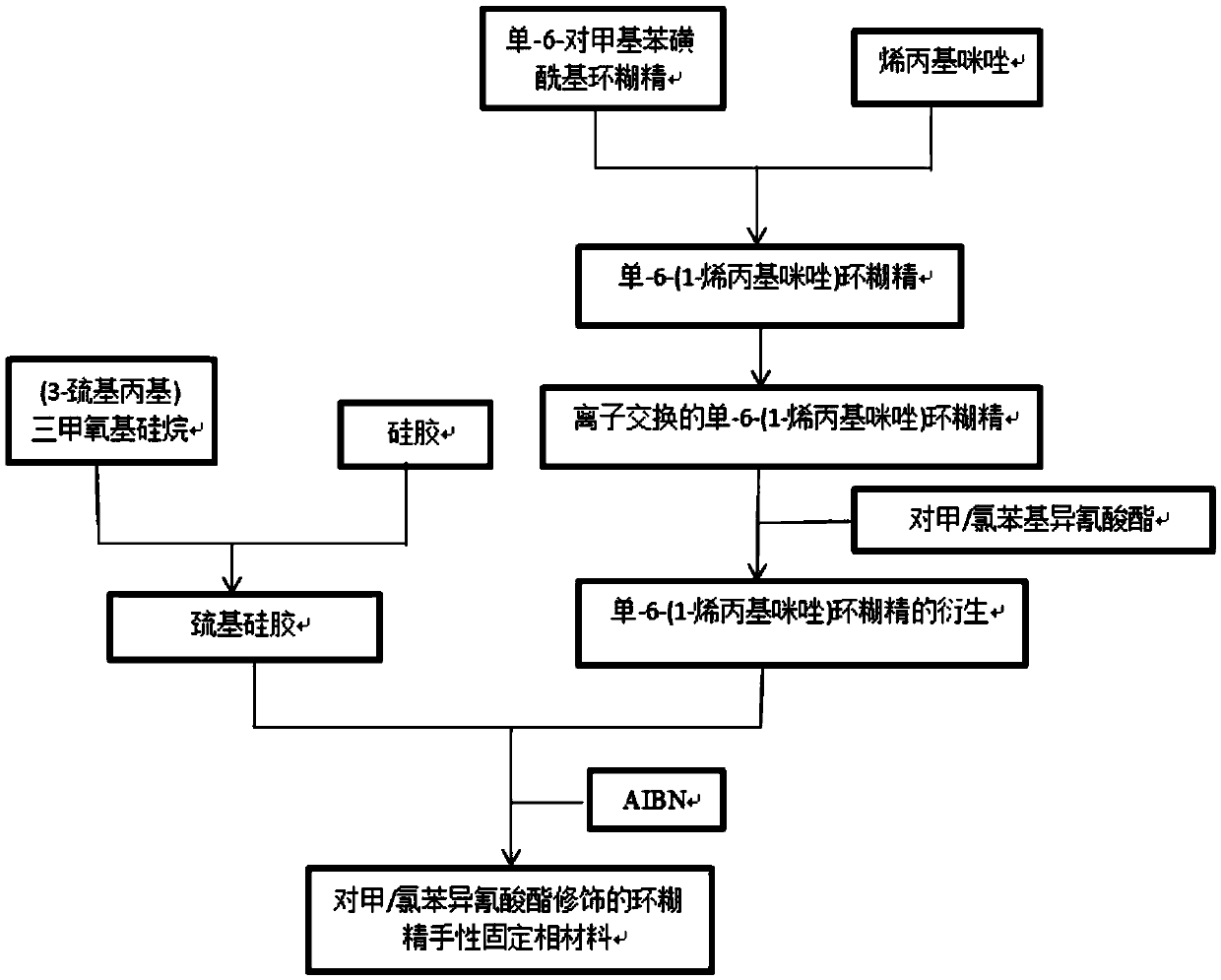
[0036] Such as figure 1 Shown in the present invention, the preparation method of the cationic cyclodextrin chiral resolution material modified by toluene / chlorophenylisocyanate, the steps are as follows:
[0037] Step 1, the preparation of mono-6-(1-allylimidazole) cyclodextrin:
[0038] Add mono-6-p-methylbenzenesulfonyl cyclodextrin and 1-allyl imidazole in an organic solvent, the organic solution used is toluene, ethanol, acetonitrile, pyridine, N,N-dimethylformamide, di One of the methyl sulfoxides. The mono-6-p-toluenesulfonyl cyclodextrins used were mono-6-p-toluenesulfonyl-α-cyclodextrin, mono-6-p-toluenesulfonyl-β-cyclodextrin and One of the mono-6-p-toluenesulfonyl-γ-cyclodextrins. The molar ratio of the mono-6-p-methylbenzenesulfonyl cyclodextrin to 1-allylimidazole is 1:2 to 1:5, react at 50 to 150°C for 20 to 40 hours, and then the reaction system Pour into acetonitrile to precipitate a solid, wash twice with acetonitrile, and dry to obtain mono-6-(1-allylimid...
Embodiment 1
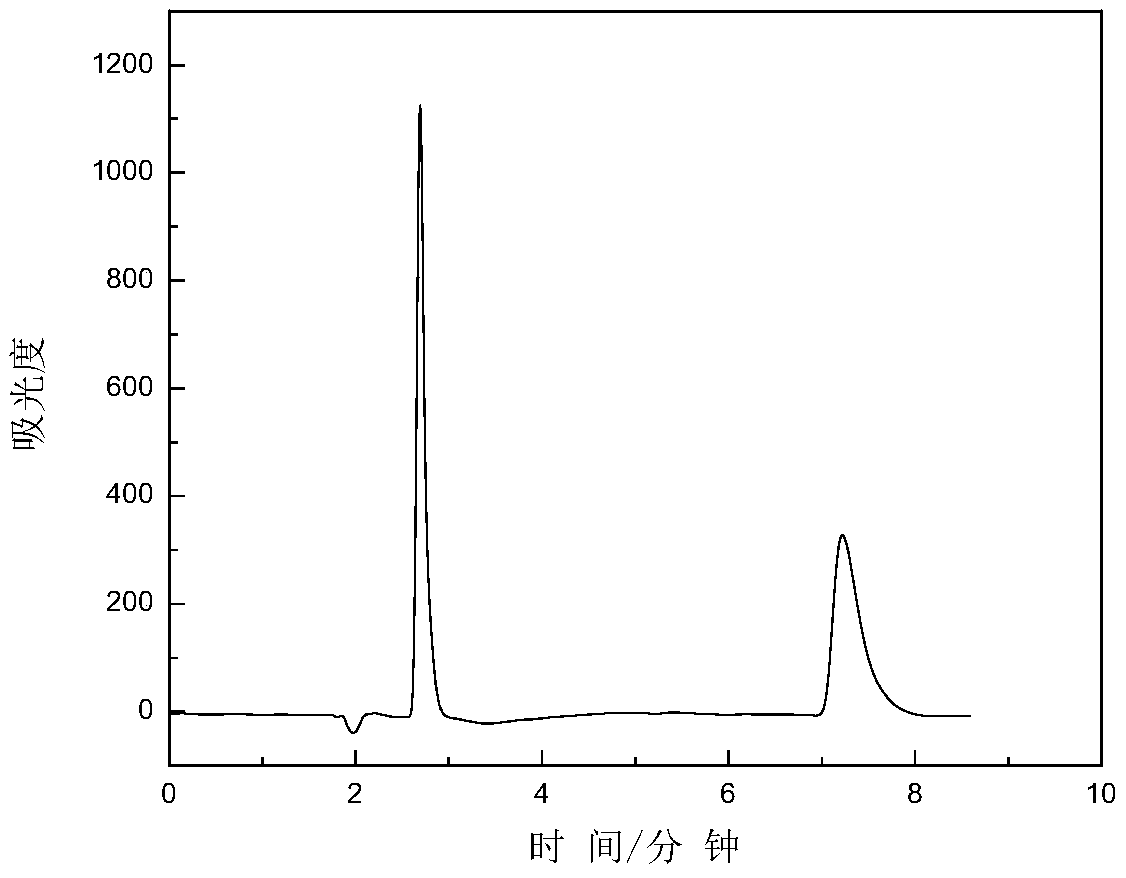
[0059] 5 g of mono-6-p-toluenesulfonyl-β-cyclodextrin, 20 mL of DMF and 2 mL of 1-allyl imidazole were sequentially added into a 100 mL three-necked flask, and the reaction was stirred at 90° C. for 36 hours. Acetonitrile was added, and a large amount of white solid was precipitated, which was filtered by suction and dried in vacuum at 60° C. for 3 hours. Mono-6-(1-allyl imidazole)-β-cyclodextrin was obtained.
[0060] Dissolve mono-6-(1-allylimidazolium)-β-cyclodextrin in a 300mL beaker, install the chloride-type anion resin in an ion-exchange column, and wash it with water continuously until the resin is neutral. The mono-6-(1-allylimidazole)-β-cyclodextrin solution was poured into an ion exchange column, rinsed continuously with a large amount of water, and the obtained filtrate was spin-dried with a rotary evaporator to obtain the product.
[0061] Add 2.14 g of mono-6-(1-allylimidazole)-β-cyclodextrin, 50 mL of pyridine, and 12 mL of p-toluene / chlorophenyl isocyanate int...
Embodiment 2
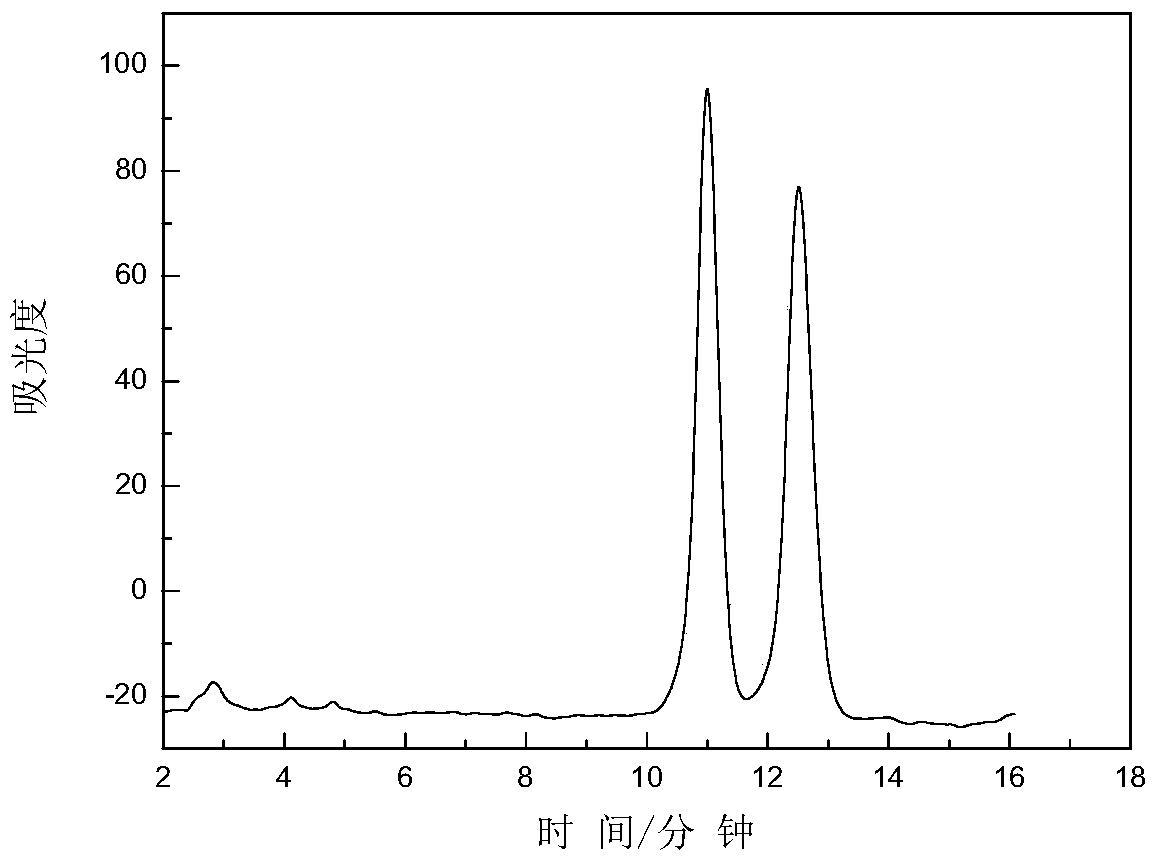
[0069] Add 10.5g of mono-6-p-toluenesulfonyl-β-cyclodextrin, 15mL of DMF and 3.5mL of 1-allyl imidazole to a 250mL three-necked flask in sequence, and stir at 70°C for 24 hours. Acetonitrile was added, and a large amount of white solid was precipitated, which was filtered by suction and dried in vacuum at 60° C. for 3 hours. Mono-6-(1-allyl imidazole)-β-cyclodextrin was obtained.
[0070] Dissolve mono-6-(1-allylimidazole)-β-cyclodextrin in a 100mL beaker, install the nitrate anion resin in an ion exchange column, and wash it with water continuously until the resin is neutral. The mono-6-(1-allylimidazole)-β-cyclodextrin solution was poured into an ion exchange column, rinsed continuously with a large amount of water, and the obtained filtrate was spin-dried with a rotary evaporator to obtain the product.
[0071] Add 1.5 g of mono-6-(1-allylimidazole)-β-cyclodextrin, 30 mL of pyridine, and 5.5 mL of p-toluene / chlorophenyl isocyanate into a 100 mL three-necked flask. N 2 pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com