lag-3 affinity peptide n13, preparation method and application thereof
A LAG-3, affinity technology, applied in the field of anti-tumor application, preparation, LAG-3 affinity peptide N13, can solve problems such as poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
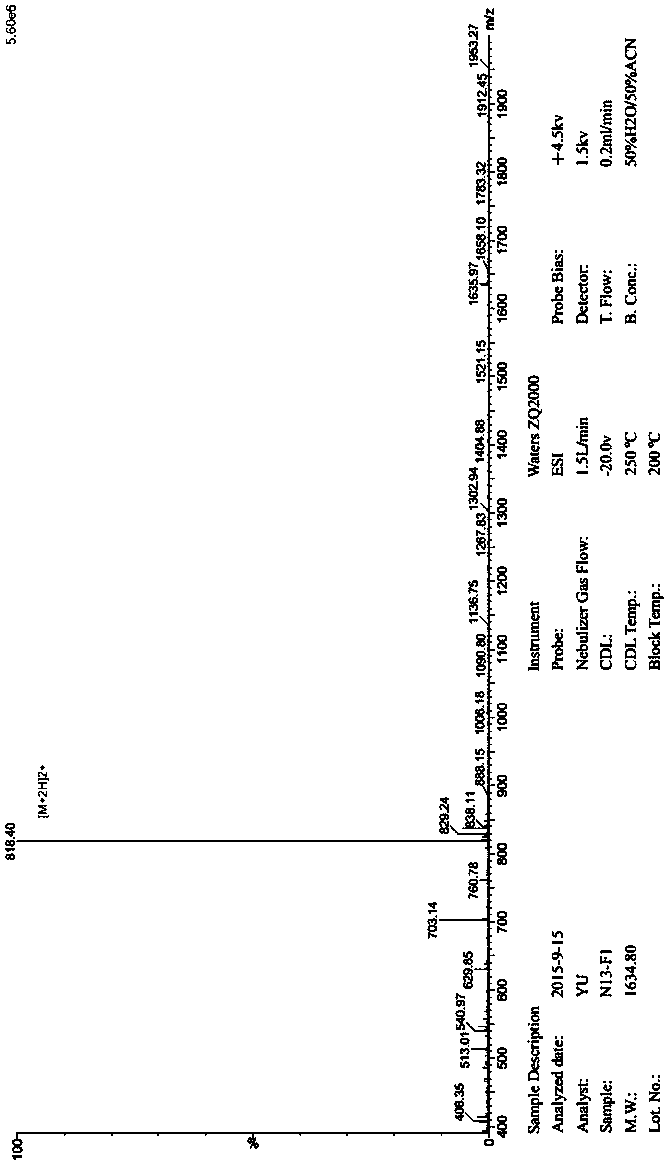
[0037] This example mainly briefly introduces the process of screening and obtaining LAG-3 affinity peptide N13.
[0038] The LAG-3 affinity peptide N13 in the present invention is mainly obtained by liquid-phase and solid-phase reverse panning methods for phage display dodecapeptide library screening, and its main technical process is: binding to the target protein→amplification→binding to the target protein → Elution. After 3-5 rounds of screening, phage monoclonals with affinity to the extracellular segment of the target protein LAG-3 were enriched round by round, and the recovery rate was greatly improved. Then, the blue spots were selected from the third round and the fifth round for sequencing, and a plurality of different inserted dodecapeptide sequences, ie, affinity peptide sequences, were obtained respectively, among which the N13 peptide was shared by the two screening methods. The specific process of screening is described below.
[0039] The first thing that nee...
Embodiment 2
[0089] This example mainly briefly introduces the artificial synthesis preparation method of LAG-3 affinity peptide N13 as follows.
[0090] The preparation method of LAG-3 affinity peptide N13 is prepared by Fmoc solid-phase peptide synthesis method. The technical principle is: select Rink resin to connect with the first Fmoc-amino acid carboxyl group at the C-terminal of the peptide to be synthesized in the form of a covalent bond, Then use the N-terminal of the amino acid as the starting point for the synthesis of the polypeptide, and allow it to undergo a dehydration condensation reaction with the carboxy-terminal of the next amino acid to form a peptide bond; then, deprotect the protecting group of the Fmoc-amino acid at the N-terminal, and then Let the N-terminal of the second amino acid react with the carboxyl group of the following amino acid; repeat this process until the synthesis of the polypeptide is completed; finally, the synthetic polypeptide is cut off from the ...
Embodiment 3
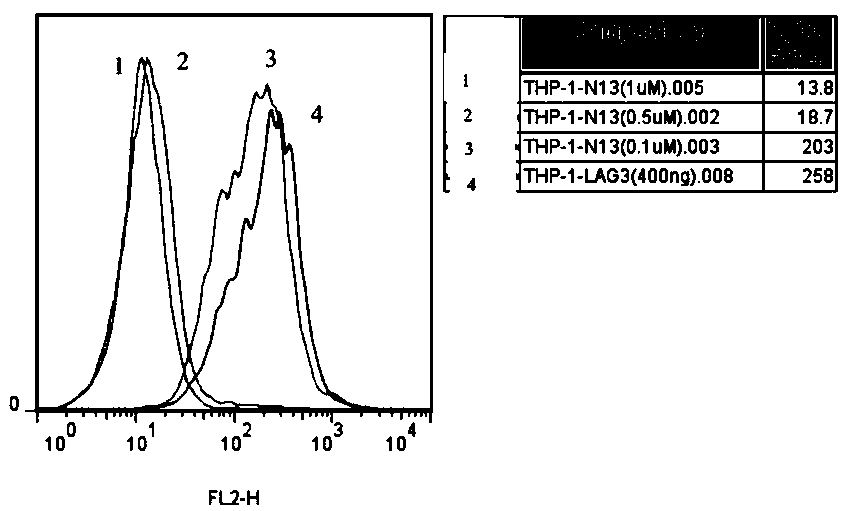
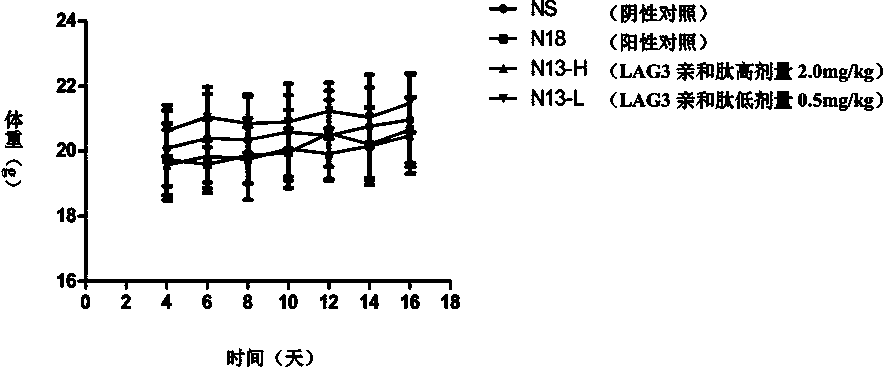
[0120] Taking the LAG-3 affinity peptide N13 prepared in Example 2 as an example, this example mainly conducted an in vitro blocking experiment on the affinity effect of the LAG-3 affinity peptide N13 and LAG-3, and the results showed that it can block LAG3 / MHC-II signaling pathway, related experiments are introduced as follows.
[0121] (1) THP-1 cells were stimulated with 20ng / mL IFN-r for 12h, and then incubated on ice with Anti-Human HLA-DR APC flow antibody, and then the ligand HLA-DR molecules on the surface were detected by flow cytometry The expression level reaches the highest level, and the cells are in good condition;
[0122] (2) Incubate the above stimulated THP-1 cells with different concentrations of LAG3-Fc fusion protein, then incubate Anti-human IgG1-Fc PE antibody on ice, and use flow cytometry to detect the surface of the above stimulated THP-1 cells The binding amount of LAG3-Fc fusion protein is about 400ng;
[0123] (3 Dissolve the affinity peptide N13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com