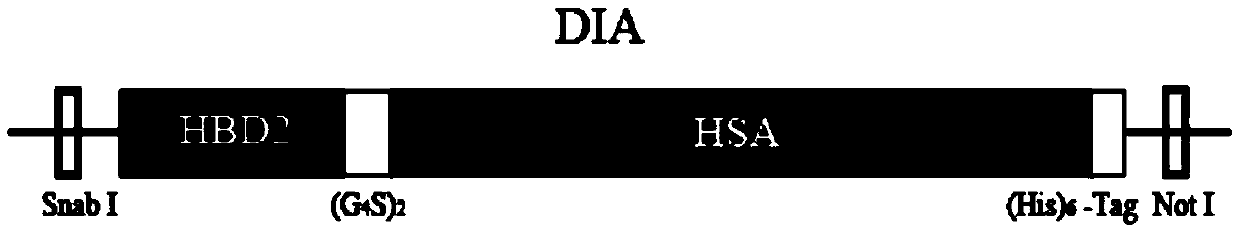
Defensin-albumin anti-tumor fusion protein and preparation and application thereof
A technology of fusion protein and defensin, which is applied in the field of anti-tumor fusion protein, can solve the problems of non-immunogenicity and achieve remarkable therapeutic effect, good application prospect and good targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] "Example 1" construction of recombinant expression vector pPIC9K-DIA
[0049] The amino acid sequences of the defensins HBD2 and HSA constituting DIA are from GenBank:
[0050] AAN64161.1 and CAA00047.1, processed by OptimumGeneTM codon optimization technology, were synthesized by Nanjing GenScript Co., Ltd. to provide plasmids PUC57s-HBD2 and PUC57s-HSA containing HBD2 and HSA coding genes. PCR primers used in molecular biology experiments were
[0051] Synthesized by InvitrogenTM Company, the plasmid pPIC9K was purchased from Invitrogen Company, and the Escherichia coli competent DH5α was a product of Beijing Quanshijin Biotechnology Company.
[0052] Primers for constructing recombinant pPIC9K-DIA:
[0053] β2F2:TCTG TACGTA GGTATTGGTGACCCTGTTACTTGTTTGAAGTCCGGTGCTATTTG54bp (the underline is the SnabI enzyme site)
[0054] β2R2: CTTGTGTGCATCGGAACCTC CACCTCCAGAACCTCCACCTCCAGGTTTCT TGCAACACT59bp (the underlined reverse complementary sequence is (G4S)2 connecting p...
Embodiment 2
[0062] 《Example 2》Induced expression, purification and identification of DIA
[0063] The GS115-DIA strain was induced and expressed on a small scale, and high-expression strains were screened out. The main steps are: pick the single colonies grown on the MD plate and inoculate them into BMGY medium (1% yeast extract, 2% peptone, 1.34% YNB, 4×10 -5 %biotin, 100mM pH6.0 potassium phosphate buffer, 1% glycerol), 28-30°C, 280-300rpm, cultivated to logarithmic phase (OD 600 =2-6). Transfer 0.5mL of culture solution to 50mL of BMGY medium (500mL shake flask, bottled at ≤10%), culture at 28-30°C, 280-300rpm, for 24-36h. Centrifuge at room temperature at 3,000×g for 5 minutes, discard the supernatant, collect the bacteria, and transfer the cell pellet to 50 mL of BMMY medium (1% yeast extract, 2% peptone, 1.34% YNB, 4×10 -5 % biotin, 100mM pH6.0 potassium phosphate buffer, 1% methanol), 28°C, 280-300rpm, cultured for 96h (100% methanol was added every 24h to a final concentration ...
Embodiment 3
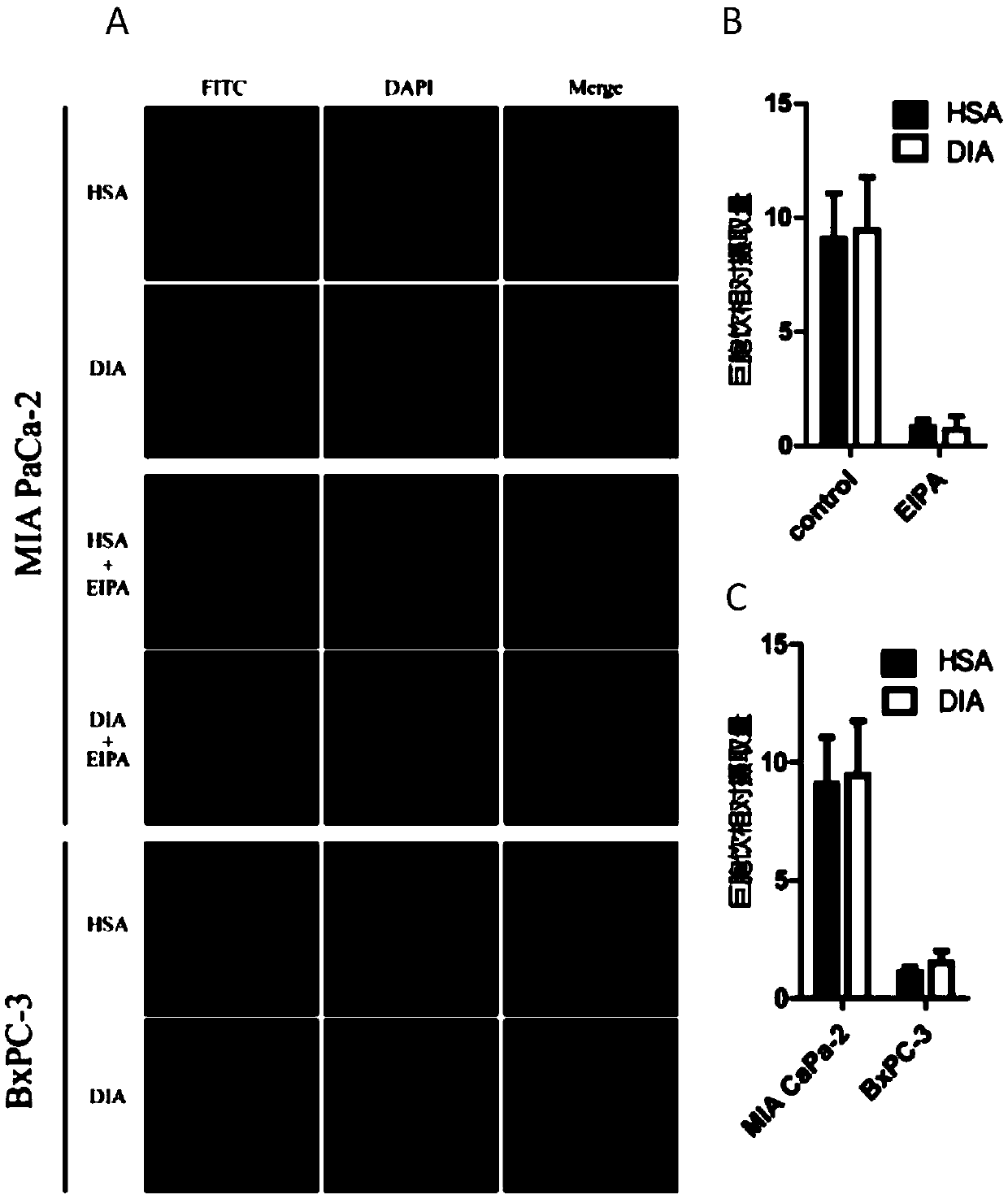
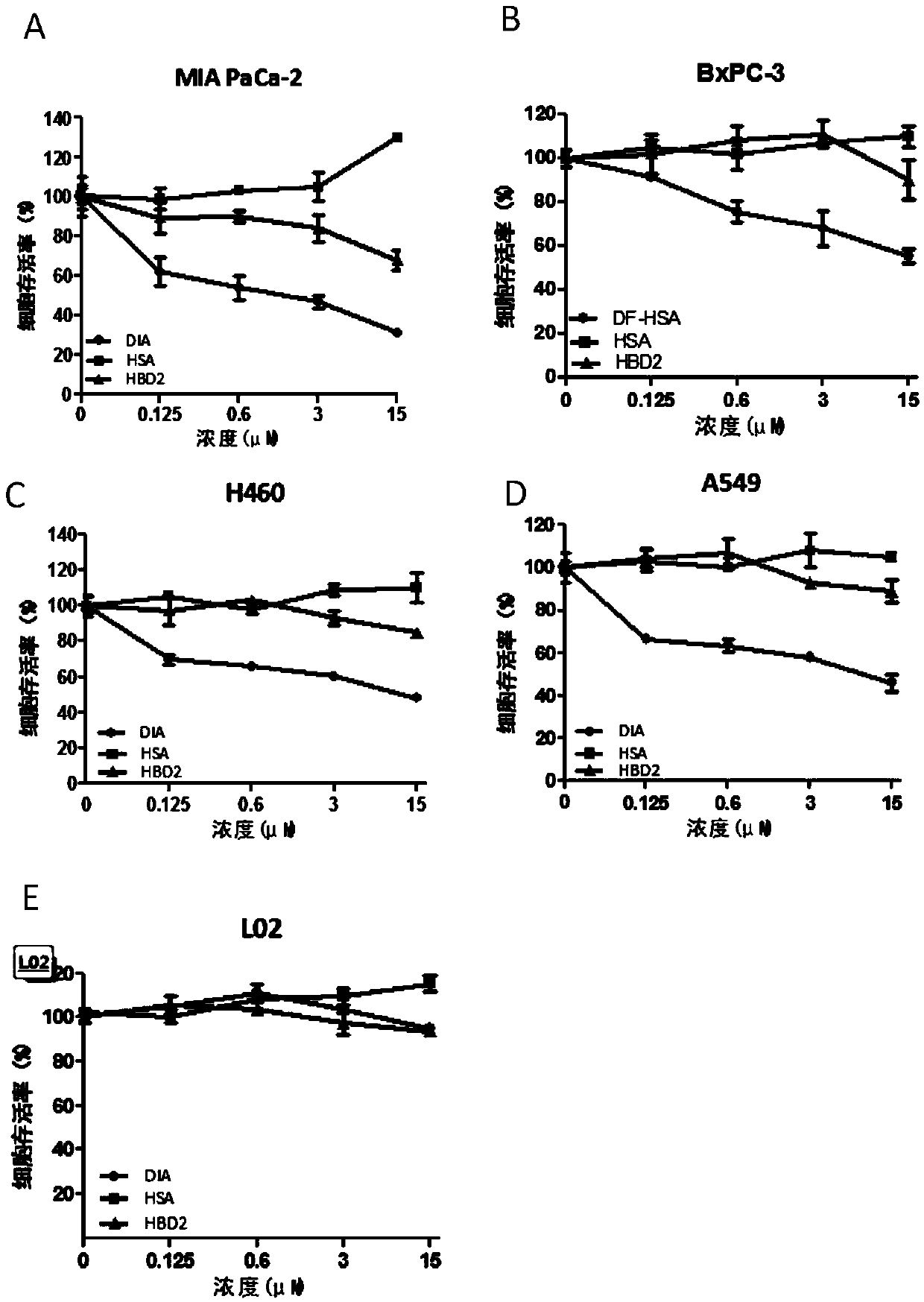
[0064] "Example 3" Observation and quantification of DIA uptake by tumor cells through macropinocytosis
[0065] It has been reported that pancreatic cancer KRAS mutant MIAPaCa2 cells have significantly enhanced macropinocytosis compared with KRAS wild-type pancreatic cancer tumor cell BxPC-3. In this experiment, the pancreatic cancer cells MIAPaCa-2 and BxPC-3 in the logarithmic growth phase were made into cell suspensions, and the density was adjusted by counting the cells at 1×10 cells per well. 5 Cells were inoculated into a 12-well plate (with a sterilized round cover glass placed at the bottom of the plate), and routinely cultured until the cell confluency was about 70%. Aspirate and discard the culture medium, rinse twice with PBS, and incubate with FITC-labeled DIA protein or HSA protein (1mg / mL) at 37°C in the dark for 1-2h, and set the control group and the macropinocytosis inhibitor EIPA (amilor Pros: It is a specific inhibitor of macropinocytosis) group. Rinse wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com