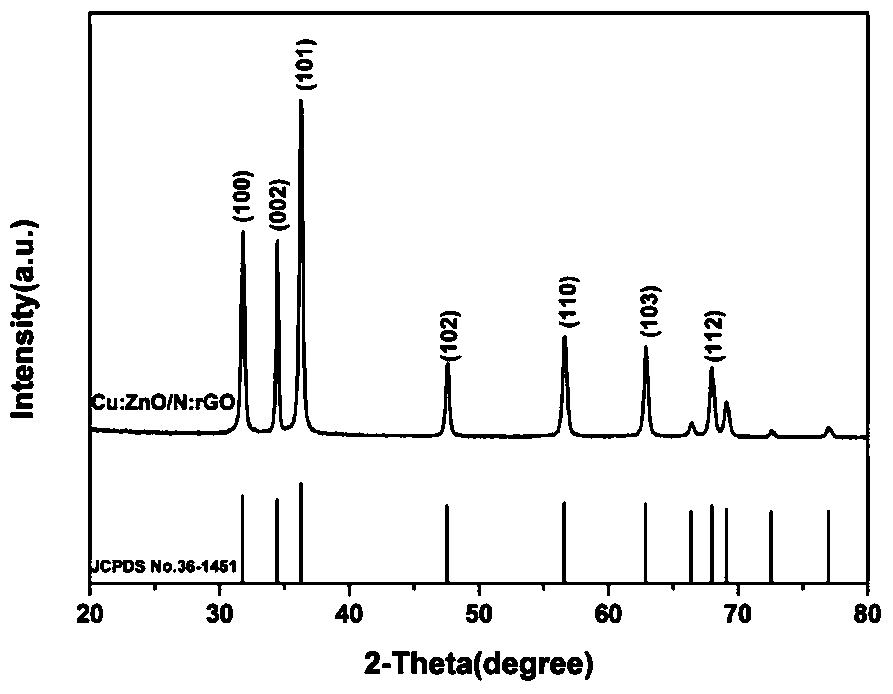
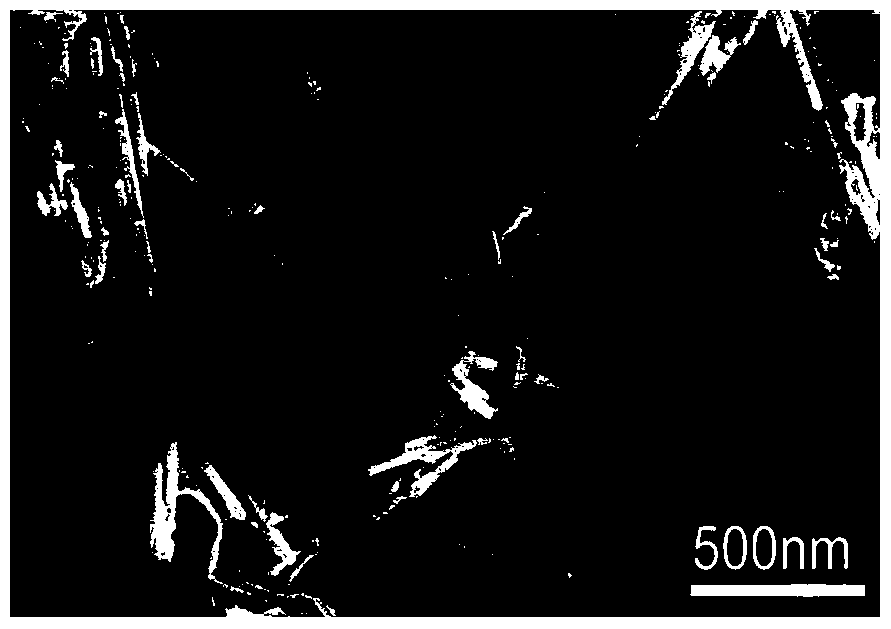
A kind of preparation method of cu:zno/n:rgo composite photocatalyst
A catalyst and composite light technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve high photocatalytic activity, high photocatalytic degradation activity, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) 0.659g Zn(CH 3 COOH) 2 2H 2 O, 0.42g HMTA, 6mg Cu(CH 3 COO) 2 2H 2 O was added to 100ml of deionized water, continuously stirred until fully dissolved, 70ml of the mixed solution was placed in a 100ml reaction kettle and kept at 90°C for 4h, and the obtained precipitate was centrifuged and dried to obtain Cu-doped ZnO nanorods;
[0023] (2) Disperse the Cu-doped ZnO nanorods prepared in step (1) in deionized water, then add 5wt% GO solution, stir well for 1h, transfer the mixed solution to a hydrothermal kettle at 120°C for 12h , and then the obtained precipitate was centrifuged and dried to obtain Cu:ZnO / rGO powder.
[0024] (3) The Cu:ZnO / rGO powder prepared in step (2) is placed in a quartz boat, and the NH 3 10% NH by volume 3 Mixed gas with Ar, then heated up to 200°C at a rate of 20°C / min, and then raised to 500°C at a constant speed within 5 minutes, and then cooled to room temperature with the furnace, and the sample was taken out to obtain a Cu:ZnO / N...
Embodiment 2
[0033] (1) 0.659g Zn(CH 3 COOH) 2 2H 2 O, 0.42g HMTA, 12mg Cu(CH 3 COO) 2 2H 2 O was added to 100ml of deionized water, continuously stirred until fully dissolved, 70ml of the mixed solution was placed in a 100ml reaction kettle and kept at 90°C for 4h, and the obtained precipitate was centrifuged and dried to obtain Cu-doped ZnO nanorods;
[0034] (2) Disperse the Cu-doped ZnO nanorods prepared in step (1) in deionized water, then add 5wt% GO solution, stir well for 1h, transfer the mixed solution to a hydrothermal kettle at 120°C for 12h , and then the obtained precipitate was centrifuged and dried to obtain Cu:ZnO / rGO powder.
[0035] (3) Place the Cu:ZnO / rGO powder prepared in step (2) in a quartz boat and pass through NH for 5 min 3 10% NH by volume 3Mixed gas with Ar, then heated up to 200°C at a rate of 20°C / min, then raised to 300°C at a constant speed within 5 minutes, and then cooled to room temperature with the furnace, and the sample was taken out to obtain ...
Embodiment 3
[0037] (1) 0.659g Zn(CH 3 COOH) 2 2H 2 O, 0.42g HMTA, 18mg Cu(CH 3 COO) 2 2H 2 O was added to 100ml of deionized water, continuously stirred until fully dissolved, 70ml of the mixed solution was placed in a 100ml reaction kettle and kept at 90°C for 4h, the obtained precipitate was centrifuged and dried to obtain Cu-doped ZnO nanorods;
[0038] (2) Disperse the Cu-doped ZnO nanorods prepared in step (1) in deionized water, then add 5wt% GO solution, stir well for 1 hour, then transfer the mixed solution to a hydrothermal kettle at 120°C for 12 hours , and then the obtained precipitate was centrifuged and dried to obtain Cu:ZnO / rGO powder.
[0039] (3) Place the Cu:ZnO / rGO powder prepared in step (2) in a quartz boat and pass through NH for 5 min 3 10% NH by volume 3 Mixed gas with Ar, then heated up to 200°C at a rate of 20°C / min, then raised to 400°C at a constant speed within 5 minutes, and then cooled to room temperature with the furnace, and the sample was taken out...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com