Fluorescent probe GH and preparation method and application thereof
A fluorescent probe and fluorescence technology, applied in the field of fluorescent probes, can solve the problem of few fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 (synthesis of probe):
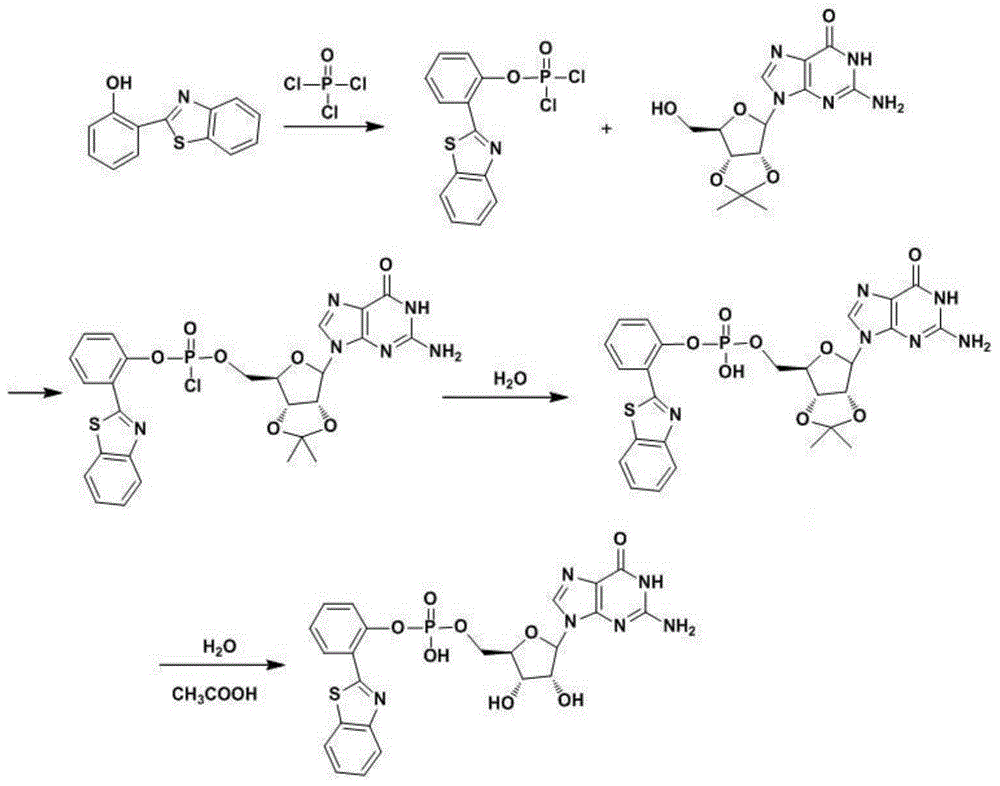
[0032] Such as figure 1 As shown, the synthesis of GH: a 50mL two-necked flask was replaced with vacuum / nitrogen three times, 20mL of dry pyridine and 2mL of dry phosphorus oxychloride were added, HBT1.81g was added slowly, and stirred at room temperature for 1h. After 1 h, it was heated and evaporated to dryness at 70°C under vacuum to obtain a light green solid. Stop heating and wait for cooling, reconnect the vacuum / nitrogen replacement three times, add 20 mL of pyridine again, vortex to dissolve all the light green precipitate, add 2.58 g of 2'3'-O-isopropyguanosine (2',3'-O-isopropyguanosine (2',3'-O-isopropyguanosine) Propylene guanosine), stirred, and reacted at room temperature for 10 h. After 10 hours, heat and spin dry at 70°C under vacuum. Add 20 mL of distilled water, stir for 1 h, filter with suction, and dry by infrared to obtain 2.05 g of solid. The obtained solid was washed three times with methanol (50 mL each ti...
Embodiment 2
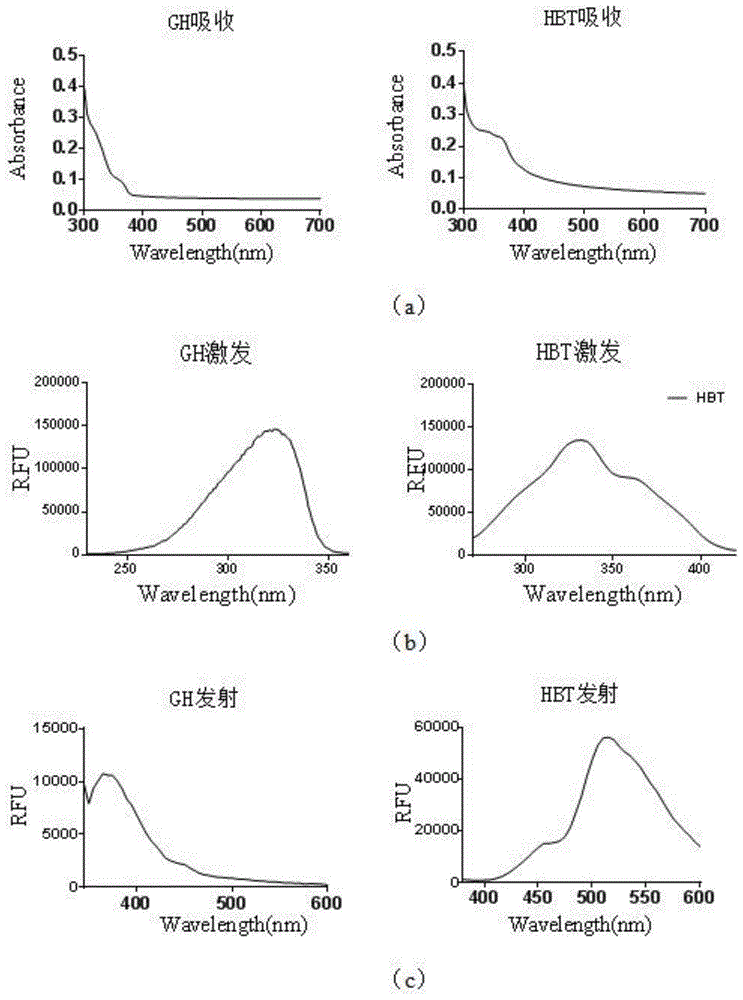
[0034] Such as Figure 4 Shown, (UV-Vis absorption spectrum (a), fluorescence excitation spectrum (b), emission spectrum (c) of fluorescent probe GH and fluorophore HBT aqueous solution):
[0035] Dissolve GH in 100 mM PB buffer to make a 20 μM solution. Take 3mL of the solution and add it to a cuvette of 1cmⅹ1cmⅹ4cm, measure the UV-visible absorption spectrum (a) of the working solution, the fluorescence excitation spectrum (b) and emission spectrum (c) of the fluorophore HBT aqueous solution produced after the action of GH and alkaline phosphatase )); The results show that the maximum excitation wavelength of the probe GH / HBT is 318nm / 330nm. The maximum emission wavelength of the probe GH is 370nm, and the fluorophore HBT has two emission peaks at 460nm / 512nm with different concentrations in aqueous solution.
Embodiment 3
[0036]Embodiment 3 (GH is to the selectivity of alkaline phosphatase):
[0037] Alkaline phosphatase, acid phosphatase, adenylyl cyclase, 3'-5' phosphodiesterase (four kinds of enzyme concentrations are all 10μM) aqueous solution, the four kinds of enzymes are divided into several parts, each part The same amount was stored at -20°C. Thaw slowly on ice before use and use each aliquot only once. Configure GH (20 mM) in DMSO (an important polar aprotic solvent). Add 2 μLGH (20 mM) and 2 μL enzyme to 196 μL of 100 mM PB buffer for each reaction, and use a full-wavelength scanning multifunctional reader and a 96-well microplate plate for measurement. The final concentrations of the probe and enzyme are 20 μM and 100 nM, respectively. Measure the fluorescence emission spectrum of the working solution, λex=330nm, the grating width is 5nm, λem=512nm.
[0038] The results of the selectivity experiment of GH to alkaline phosphatase are as follows: Figure 5 As shown, the ordinate r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com