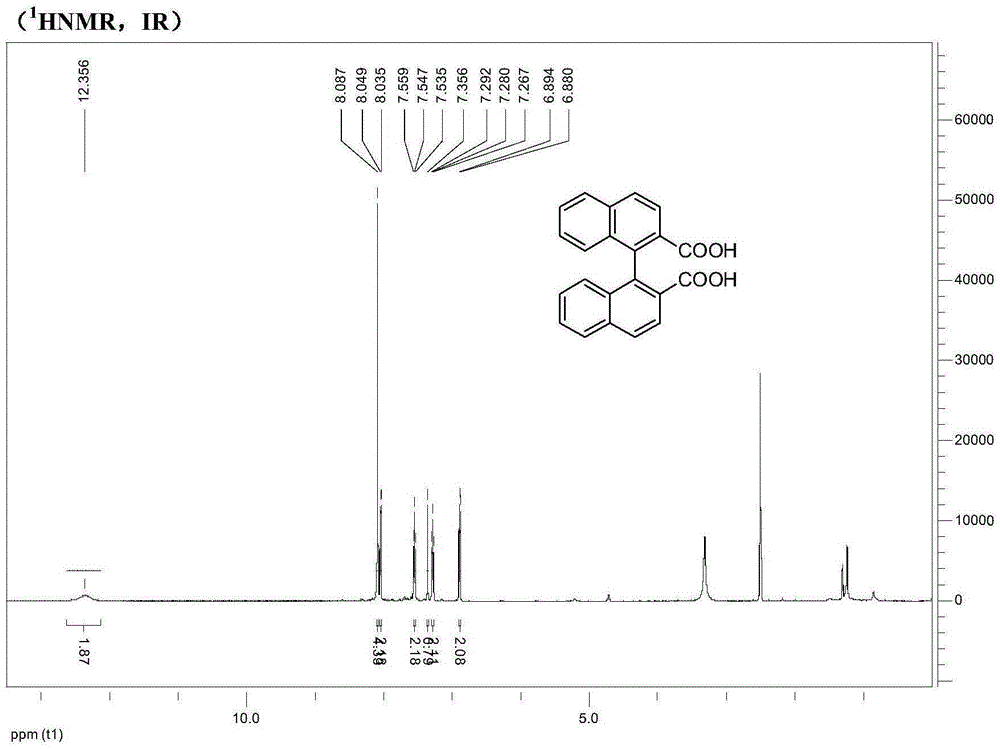
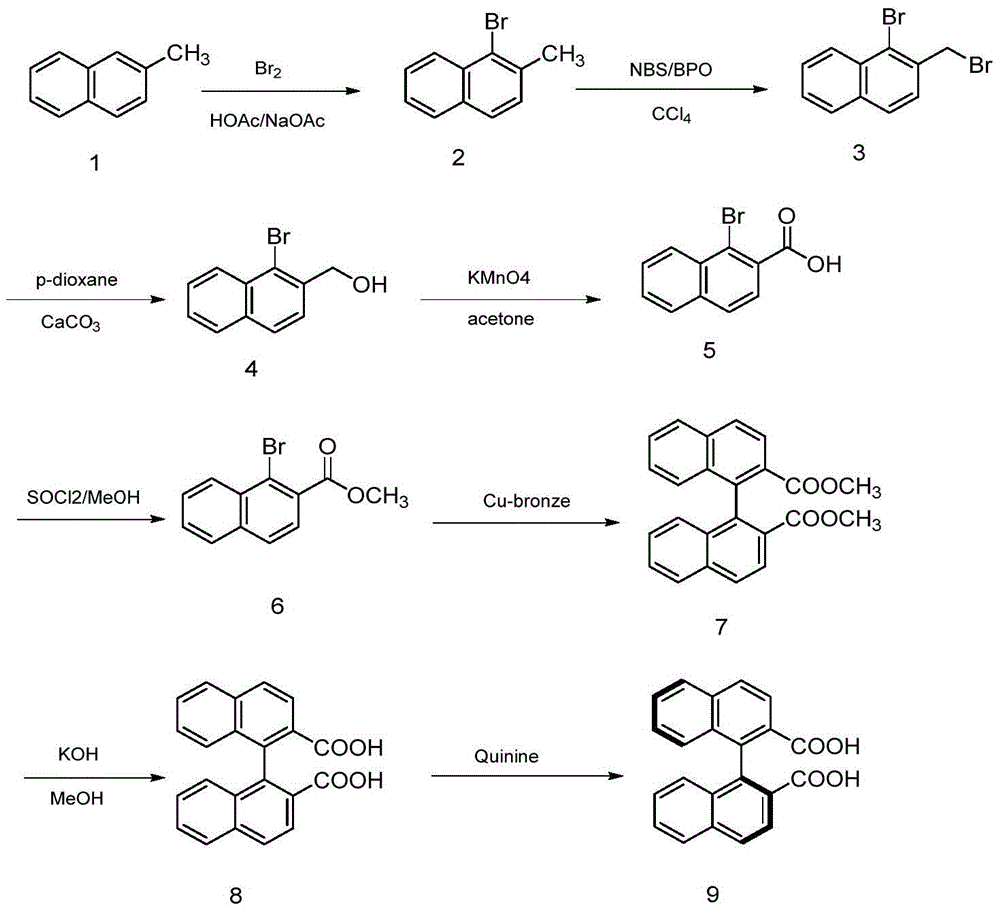
Synthesis method of axially chiral dinaphthalene ligand precursor (s)-2,2'-dinaphthyl-1,1'-dicarboxylic acid
A technology of dicarboxylic acid and axial chirality, which is applied in the fields of oxidation, hydrolysis, coupling reaction and bromination. It can solve the problems of poor economy of reagents, harsh conditions and difficulties in industrialization, and achieve high yield, simple operation, Good economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1) Add 10.05g of 2-methylnaphthalene, 6.42g of sodium acetate and 30mL of acetic acid solution in a 250mL round bottom flask. Add 20mL of acetic acid and 4mL of liquid bromine into the dropping funnel, and add them dropwise into the round bottom flask. The solution turns yellow, and continue to stir for 15 minutes to stop the reaction. After extraction, drying and concentration, 13.5 g of light yellow oily product (1-bromo-2-methylnaphthalene) was obtained with a yield of 86.3%.
[0015] 2) Add 10.2g of 1-bromo-2-methylnaphthalene and 8.2g of NBS and 100mL of CCl in a 250mL round bottom flask 4 In the solution, add 0.06g benzoyl peroxide (BPO) again, reflux and stir for 3h, then add 0.06g benzoyl peroxide (BPO), reflux for 3h, the solution is overnight at room temperature, then the suspension is heated to the boiling point, multiplied Hot filtration, filtrate directly with CCl 4 The solution was recrystallized and recrystallized three times to obtain 10.0 g of light y...
Embodiment 2
[0023] 1) In a 250mL round bottom flask, add 10.05g of 2-methylnaphthalene, 12.80g of sodium acetate and 30mL of acetic acid solution. Add 20mL of acetic acid and 4mL of liquid bromine into the dropping funnel, and add them dropwise into the round bottom flask. The solution turns yellow, and continue to stir for 15 minutes to stop the reaction. After extraction, drying and concentration, 13.7 g of light yellow oily product (1-bromo-2-methylnaphthalene) was obtained with a yield of 87.6%.
[0024] 2) Add 10.2g of 1-bromo-2-methylnaphthalene and 8.2g of NBS and 100mL of CCl in a 250mL round bottom flask 4 In the solution, add 0.06g benzoyl peroxide (BPO) again, reflux and stir for 3h, then add 0.06g benzoyl peroxide (BPO), reflux for 3h, the solution is overnight at room temperature, then the suspension is heated to the boiling point, multiplied Hot filtration, filtrate directly with CCl 4 The solution was recrystallized and recrystallized three times to obtain 10.0 g of light...
Embodiment 3
[0032] 1) Add 10.05g of 2-methylnaphthalene, 6.42g of sodium acetate and 30mL of acetic acid solution in a 250mL round bottom flask. Add 20mL of acetic acid and 4mL of liquid bromine into the dropping funnel, and add them dropwise into the round bottom flask. The solution turns yellow, and continue to stir for 15 minutes to stop the reaction. After extraction, drying and concentration, 13.5 g of light yellow oily product (1-bromo-2-methylnaphthalene) was obtained with a yield of 86.3%.
[0033] 2) Add 10.2g of 1-bromo-2-methylnaphthalene and 8.2g of NBS and 100mL of benzene solution into a 250mL round bottom flask, then add 0.06g of benzoyl peroxide (BPO), stir at reflux for 3 hours, and then add 0.06g of peroxide Benzoyl Oxygen (BPO), reflux for 3h, the solution was overnight at room temperature, then the suspension was heated to the boiling point, filtered while hot, and the filtrate was directly washed with CCl 4 The solution was recrystallized and recrystallized three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com