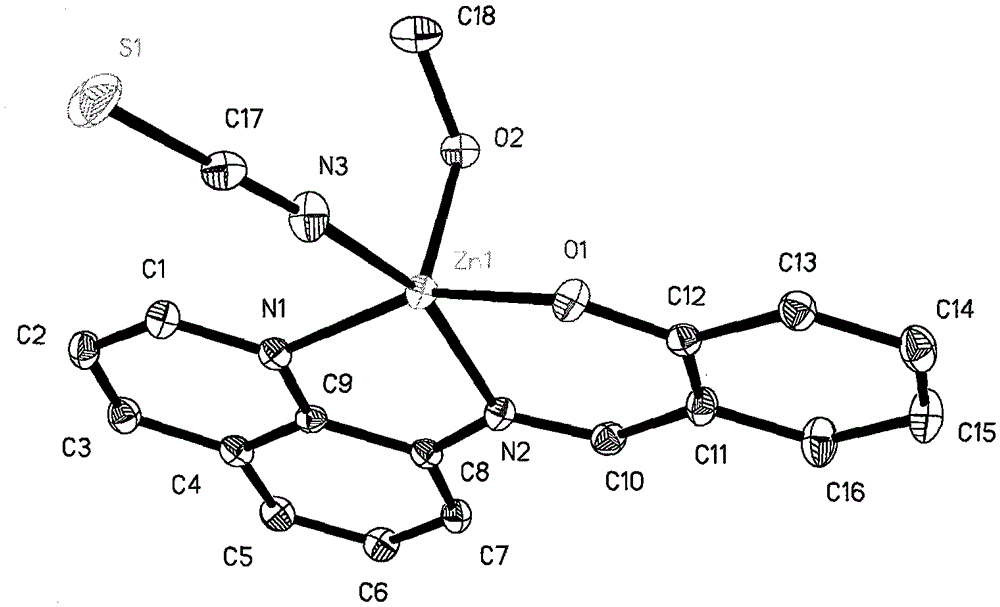
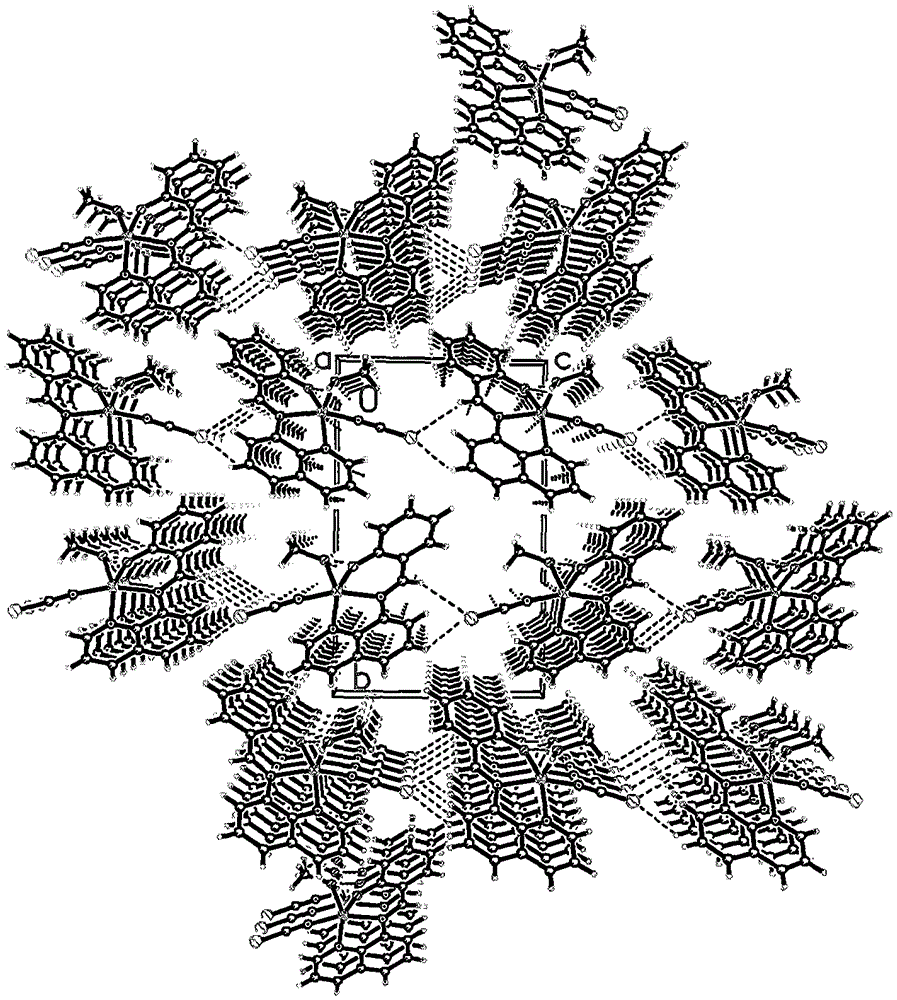
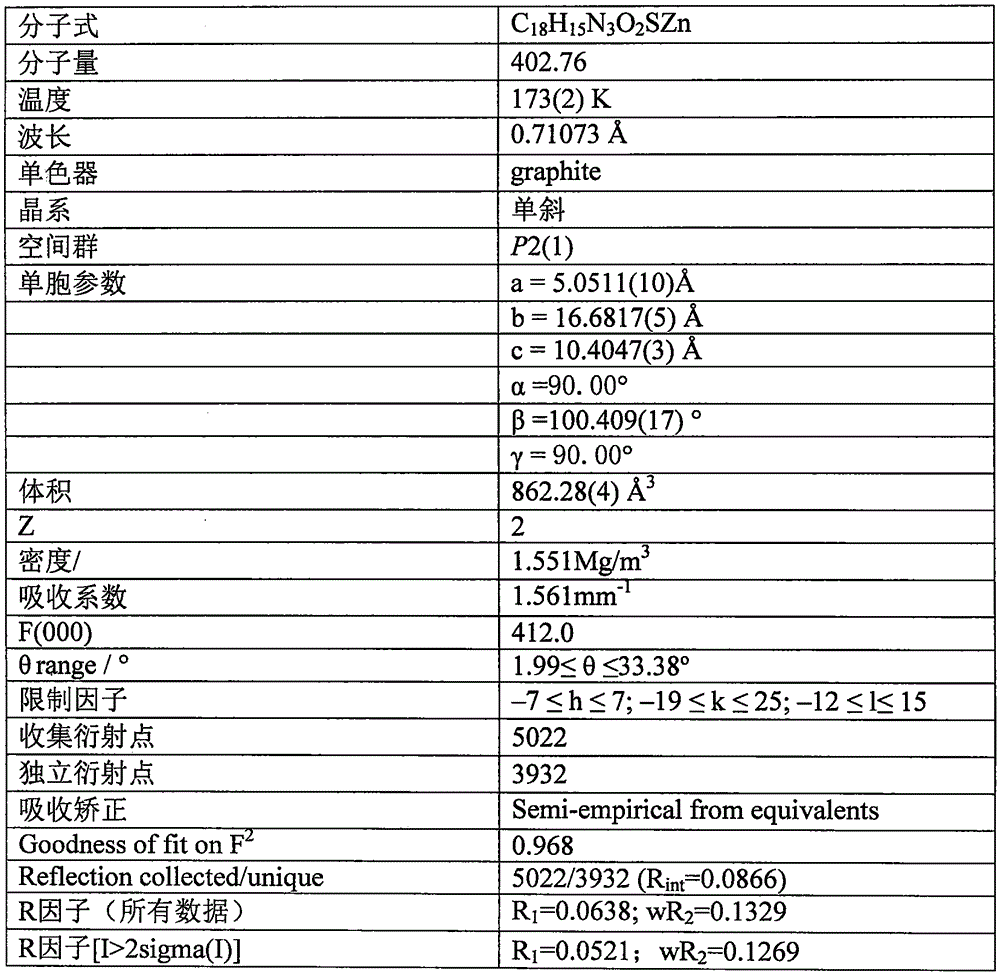
8-aminoquinoline schiff base and zinc rhodanate metal complex and preparation method thereof
A technology of aminoquinoline Schiff base zinc thiocyanate and metal complexes, applied in zinc organic compounds, organic chemical methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: 0.10 mmol of 8-aminoquinoline (14.4 mg), 0.10 mmol of salicylaldehyde (12.2 mg) and 0.10 mmol of triethylamine (10.1 mg) were all dissolved in 30 ml of methanol solvent, and refluxed for 2 hours under stirring. After cooling, slowly add 0.10mmol Zn(ClO 4 ) 2 ·6H 2 O (37.2 mg), 0.10 mmol KSCN (9.7 mg) in 10 ml of methanol solution, stirred at room temperature for 7 days, filtered; the filtrate was placed in a 50 ml beaker, sealed with a film, and small holes were made to volatilize naturally. Thirty-five days later, yellow flaky crystals were obtained, filtered, and the filter cake was washed with ether to obtain the target complex. The calculated yield was 31% based on 8-aminoquinoline.
Embodiment 2
[0014] Example 2: 0.10 mmol 8-aminoquinoline (14.4 mg), 0.10 mmol salicylaldehyde (12.2 mg) and 0.11 mmol triethylamine (11.1 mg) were all dissolved in 30 ml ethanol solvent, and refluxed for 3 hours under stirring. After cooling, slowly add 0.10mmol Zn(ClO 4 ) 2 ·6H 2 O (37.2mg), 0.12mmol KSCN (11.6mg) in 10ml ethanol solution, stirred at room temperature for 7 days, filtered; the filtrate was placed in a 50ml beaker, sealed with a film, and small holes were made to volatilize naturally. Thirty-five days later, yellow flaky crystals were obtained, filtered, and the filter cake was washed with ether to obtain the target complex. The calculated yield was 38% based on 8-aminoquinoline.
Embodiment 3
[0015] Example 3: 0.10mmol 8-aminoquinoline (14.4mg), 0.10mmol salicylaldehyde (12.2mg) and 0.12mmol triethylamine (12.1mg) were all dissolved in 30ml of acetone solvent, stirred and refluxed for 4 hours, cooled After that, slowly add 0.10mmol Zn(ClO 4 ) 2 ·6H 2 O (37.2mg), 0.15mmol KSCN (14.6mg) in 10ml of acetone solution, stirred at room temperature for 7 days, filtered; the filtrate was placed in a 50ml beaker, sealed with a film, and made small holes, and naturally volatilized. Thirty-five days later, yellow flaky crystals were obtained, filtered, and the filter cake was washed with ether to obtain the target complex. The calculated yield was 36% based on 8-aminoquinoline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com