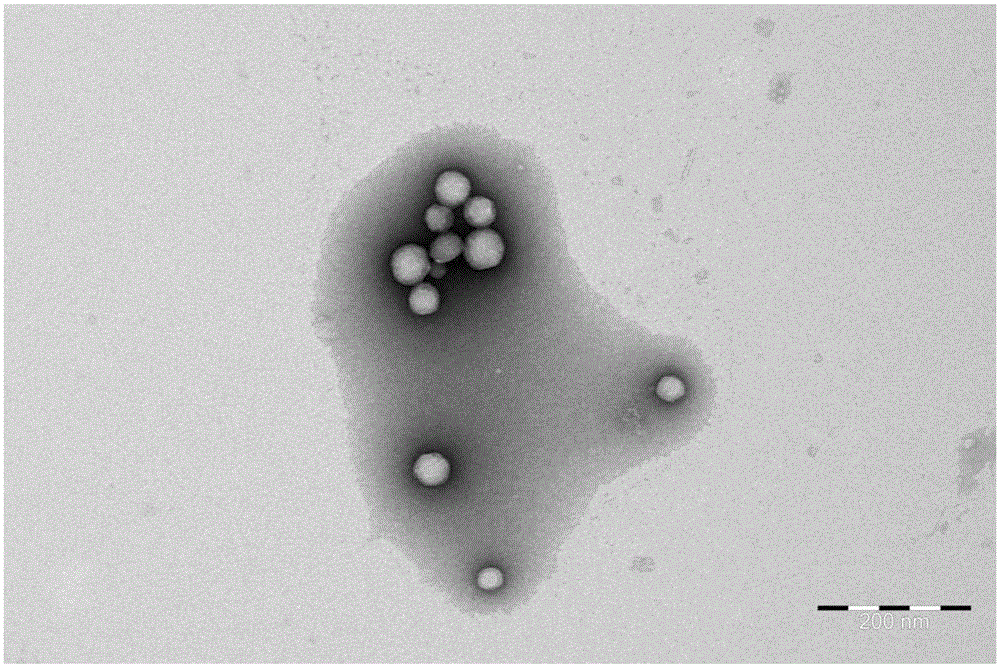
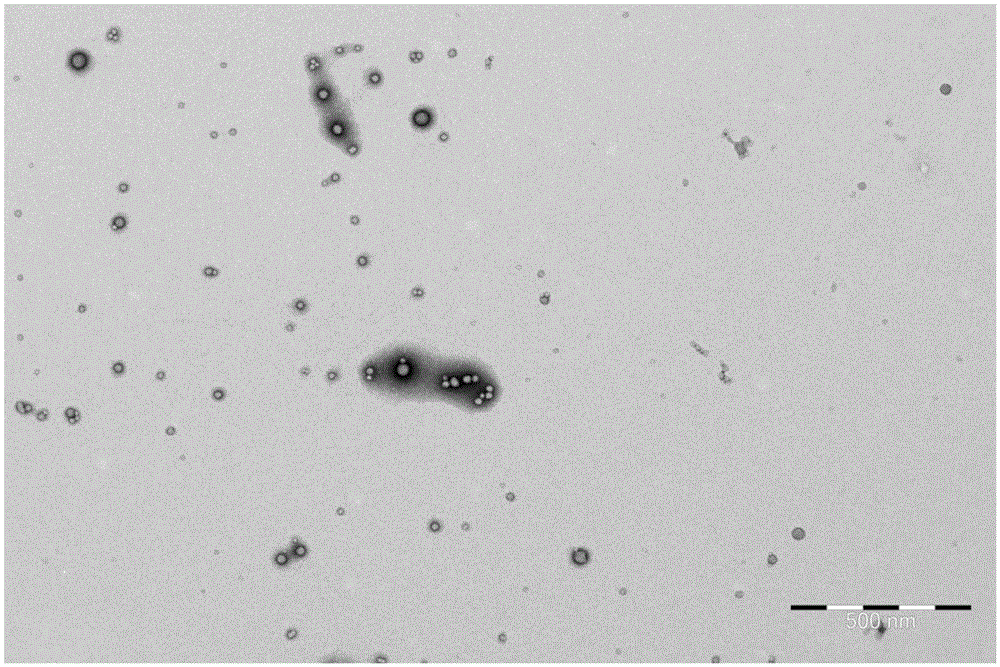
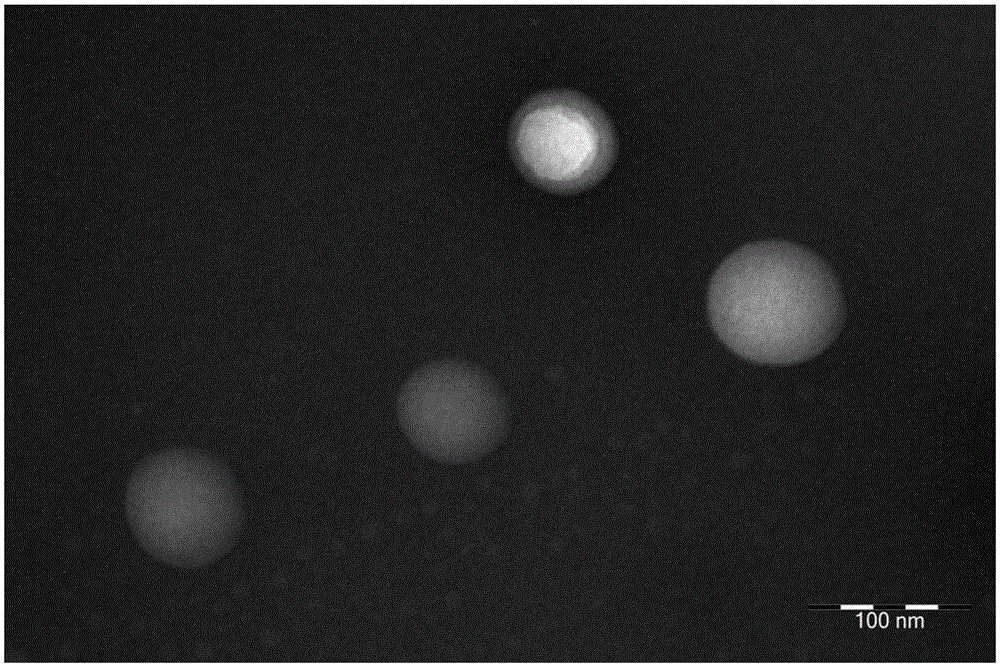
Anti-tumor medicine coupler decorated with saturated fatty acid and self-assembling nanometer system and preparation method of anti-tumor medicine coupler
A technology of anti-tumor drugs and fatty acids, which is applied in the direction of anti-tumor drugs, drug combinations, and pharmaceutical formulations, and can solve problems such as insufficient drug loading affecting drug use and development, insoluble drugs restricting drug loading, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: the synthesis of stearic acid-SN38
[0107] Under nitrogen protection, 7-ethyl-10-hydroxycamptothecin (40mg, 0.1mmol) was dissolved in an appropriate amount of anhydrous N,N-dimethylformamide, and dimethylaminopyridine (14.4mg, 0.1mmol ) and dicyclohexylcarbodiimide (24.6mg, 0.2mmol), stearic acid (29mg, 0.1mmol) was added under stirring conditions, and the stirring reaction at room temperature was continued for 12 hours. Remove the precipitate by filtration, mix the filtrate with an appropriate amount of silica gel, dry under reduced pressure and vacuum, separate and purify the sample by silica gel column chromatography, use petroleum ether: acetone (6:1) as the eluent, collect the eluate containing the sample, and blow it with nitrogen at room temperature drying or vacuum drying under reduced pressure to obtain a stearic acid-SN38 conjugate (yield 65%).
Embodiment 2
[0108] Embodiment 2: the synthesis of palmitic acid-doxorubicin
[0109] Under nitrogen protection, dissolve doxorubicin (55mg, 0.1mmol) in an appropriate amount of anhydrous dichloromethane, add O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU, 115mg, 0.15mmol) And N,N-diisopropylethylamine (100mg, 0.77mmol), then add palmitic acid (26mg, 0.1mmol), and continue stirring at room temperature for 12 hours. Remove the precipitate by filtration, mix the filtrate with an appropriate amount of silica gel, dry under reduced pressure and vacuum, separate and purify the sample by silica gel column chromatography, use petroleum ether: acetone (6:1) as the eluent, collect the eluate containing the sample, and blow it with nitrogen at room temperature Drying or vacuum drying under reduced pressure to obtain the palmitic acid-doxorubicin conjugate (yield 60%).
Embodiment 3
[0110] Embodiment 3: the synthesis of stearic acid-vinblastine
[0111] Under nitrogen protection, vinblastine (91 mg, 0.1 mmol) was dissolved in an appropriate amount of anhydrous dichloromethane, and dimethylaminopyridine (14.4 mg, 0.1 mmol) and dicyclohexylcarbodiimide (24.6 mg, 0.2 mmol), stearic acid (29mg, 0.1mmol) was added under stirring conditions, and the stirring reaction at room temperature was continued for 12 hours. Remove the precipitate by filtration, mix the filtrate with an appropriate amount of silica gel, dry under reduced pressure and vacuum, separate and purify the sample by silica gel column chromatography, use petroleum ether: acetone (6:1) as the eluent, collect the eluate containing the sample, and blow it with nitrogen at room temperature Drying or vacuum drying under reduced pressure, the palmitic acid-doxorubicin conjugate (yield 63%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com