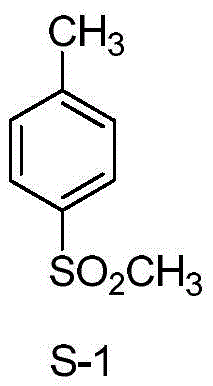
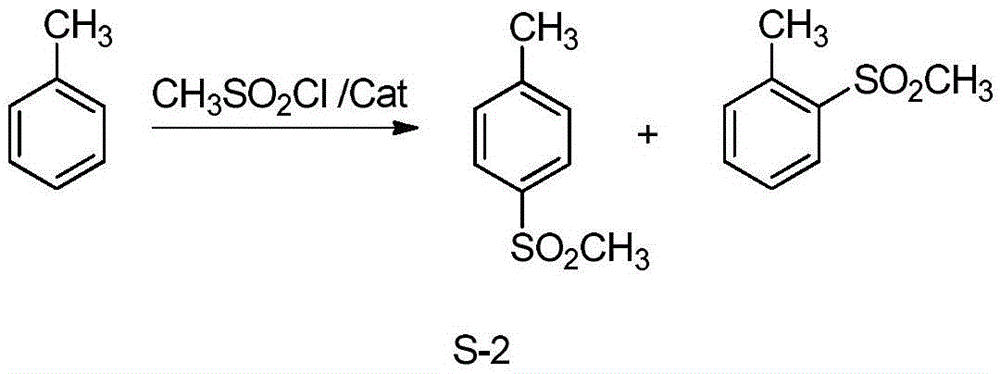
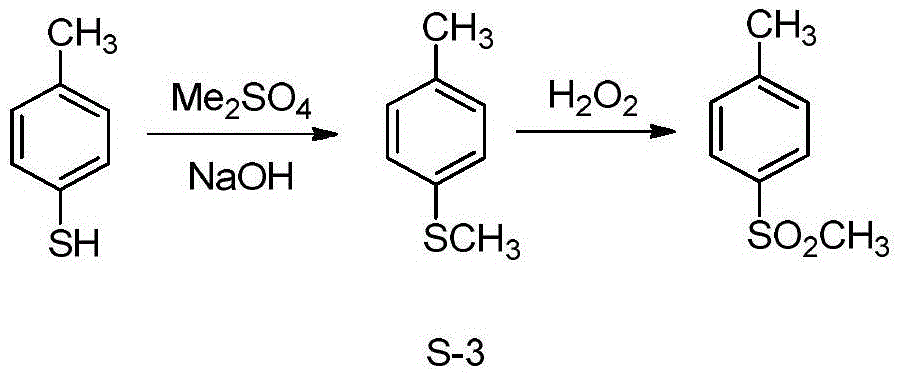
Synthetic method of 4-methylsulfonyl methylbenzene
A methanesulfonyltoluene and synthesis method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of reduced yield, poor atom economy, complicated by-products, etc., and achieve simplified production equipment and Process operation, solving the effect of poor atom economy and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, a kind of synthetic method of 4-methylsulfonyltoluene, carry out following steps successively:
[0054] 1), set two 250mL autoclave (autoclave I and autoclave II), these two autoclave respectively carry out exactly the same step 1); The molar weights of phenylsulfonyl chloride are exactly the same;
[0055] details as follows:
[0056] Add 12.6g of sodium sulfite (molecular weight 126, 0.1mol), 60mL of water, and about 16.0g of sodium bicarbonate into a 250mL autoclave with a stirring temperature measuring device to adjust the pH of the solution to 8, close the lid of the kettle, and insert condensed water ;
[0057] Use N 2 Check for leaks, turn on the stirring and heating device, and slowly raise the temperature of the reaction solution to 75-80°C; melt the raw material 4-methylbenzenesulfonyl chloride (around 70°C), and use a feed pump to feed slowly, 4.3g each time , 20min / time, feeding 4 times (a total of 17.2g, 0.09mol);
[0058] After the feedi...
Embodiment 14
[0074] Replace "60mL water, 16g sodium bicarbonate" in step 1) with "60mL water, 16g sodium bicarbonate" in the step 1) of "the filtrate II obtained by adding ice-out salt and filtering in the filtrate I" in step 5) of embodiment 1, and the remaining contents are equal to embodiment 1.
[0075] The final result obtained was: 13.2 g of the product 4-methylsulfonyltoluene was obtained, the yield was 86.3%, and the purity detected by HPLC was 99.5%.
[0076] Comparative example 1-1, change " methyl chloride " in embodiment 1 into " dimethyl sulfate " (molar weight is unchanged) carry out methylation reaction, all the other contents are equal to embodiment 1.
[0077] The final result obtained was: 8.0 g of the product 4-methylsulfonyltoluene was obtained, the yield was 52.4%, and the purity detected by HPLC was 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com