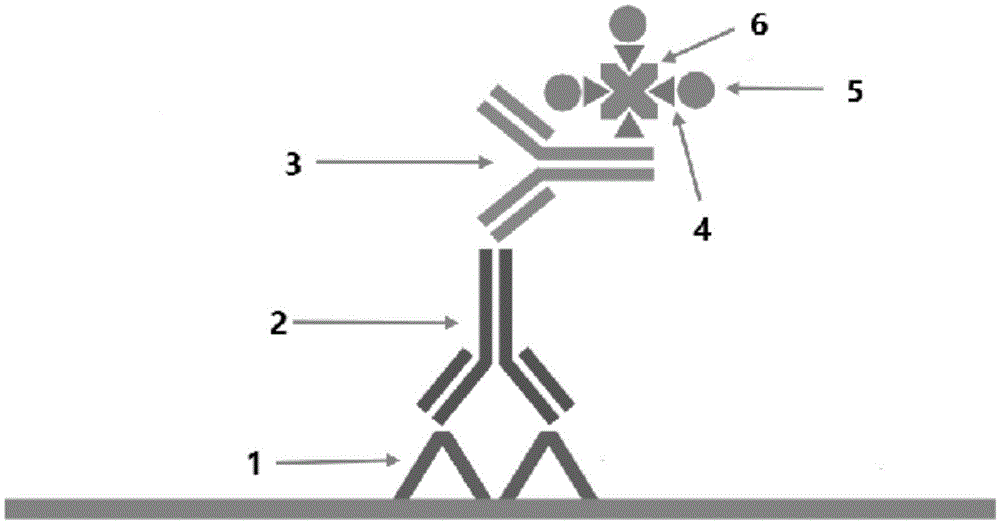
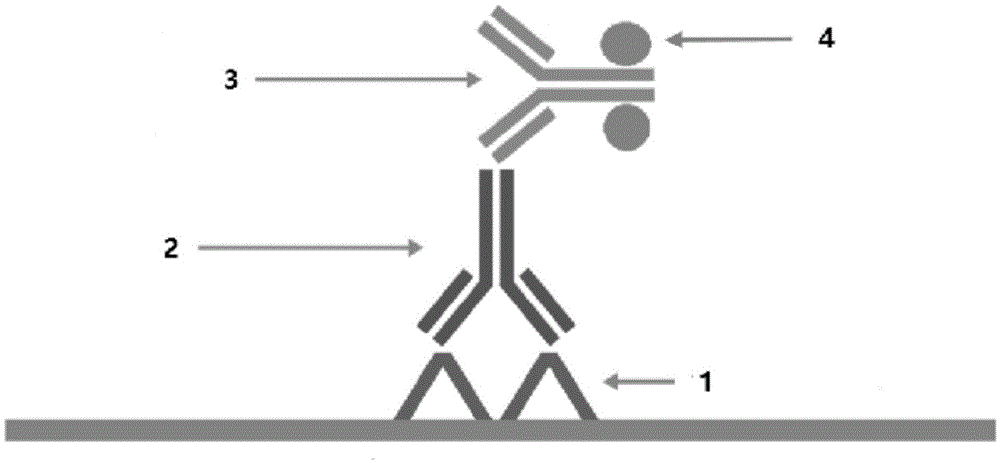
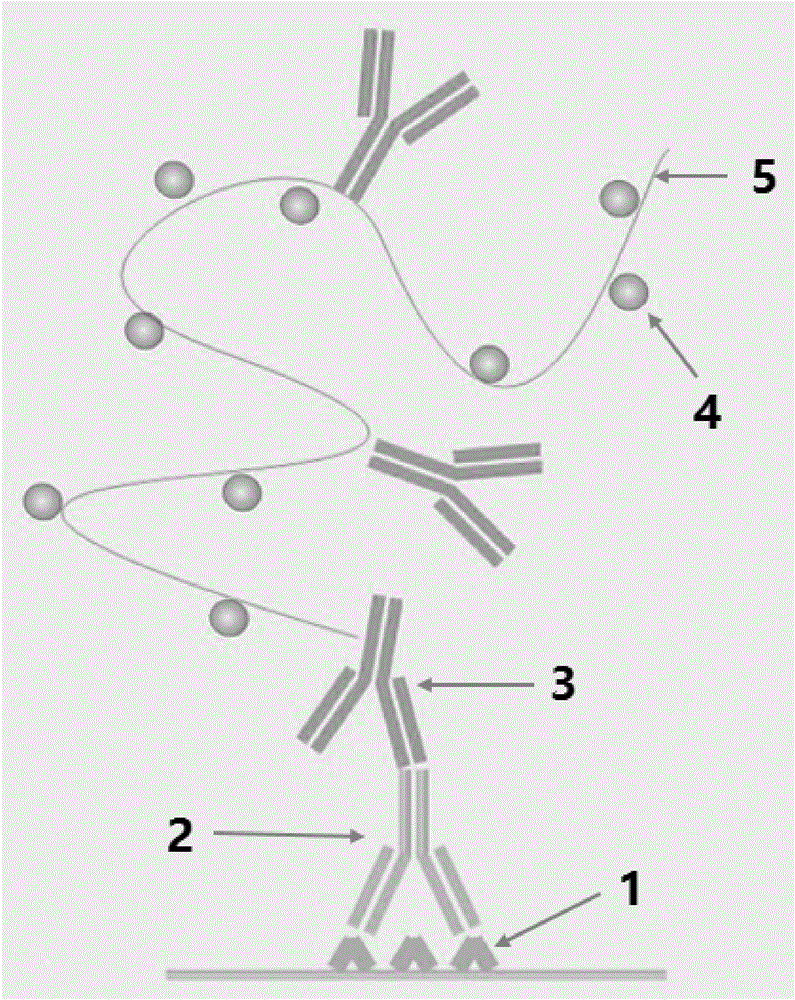
Polymer enzyme-antibody and preparation method thereof
A polymer enzyme and antibody technology, applied in the field of medical detection, can solve the problems of long incubation time, loose overall structure of the secondary antibody, misdiagnosis, etc., and achieve the effect of improving the sensitivity and improving the detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Dissolve 2,000,000U peroxidase (SigmaChemicalCompany, Catalog#No.P8375-250KU, 7.5g) in 2.0ml of sodium bicarbonate buffer (0.1M, pH8.00) at room temperature, add 0.24mL of DVS (Sigma Chemical Company, Catalog#No.V3700-5G) then gently stirred at 30°C for 30 minutes;
[0038] 2) Purify the above reaction solution through a 50 ml SephadexG-25 column (Sigma Chemical Company, Catalog No. G25150) to remove the remaining DVS, and perform the purification in sodium bicarbonate buffer (0.1M sodium bicarbonate, pH 8.0). Collect the brown fraction, which is the activating enzyme;
[0039] 3) Concentrate the above activating enzyme to 4.0ml with a centrifugal concentration tube;
[0040] 4) Add 144 mg of PAMAMDendrimer 5.0 (Sigma Chemical Company, Catalog No. 536709-5G) to the above-mentioned concentrated 4 ml of activating enzyme (the molecular ratio of activating enzyme to PANMAM is 40:1). The solution was stirred gently at 30°C for 16 hours;
[0041] 5) Purify the reaction...
Embodiment 2
[0049] 1) Dissolve 2,000,000U peroxidase (SigmaChemicalCompany, CatalogNo.P8375-250KU, 7.5g) in 2.0ml of sodium bicarbonate buffer (0.1M, pH8.00) at room temperature, add 0.24mL of DVS (SigmaChemicalCompany , CatalogNo.V3700-5G) then stirred gently at 30°C for 30 minutes;
[0050] 2) Purify the above reaction solution through a 50 ml SephadexG-25 column (Sigma Chemical Company, Catalog No. G25150) to remove the remaining DVS, and perform the purification in sodium bicarbonate buffer (0.1M sodium bicarbonate, pH 8.0). Collect the brown fraction, which is the activating enzyme;
[0051] 3) Concentrate the above activating enzyme to 4.0ml with a centrifugal concentration tube;
[0052] 4) Add 144 mg of PAMAMDendrimer 5.0 (Sigma Chemical Company, Catalog No. 536709-5G) to the above-mentioned concentrated 4ml of activating enzyme (the molecular ratio of activating enzyme to PANMAM is 40:1), and put the solution in Stir gently for 16 hours at 30°C;
[0053] 5) Purify the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com