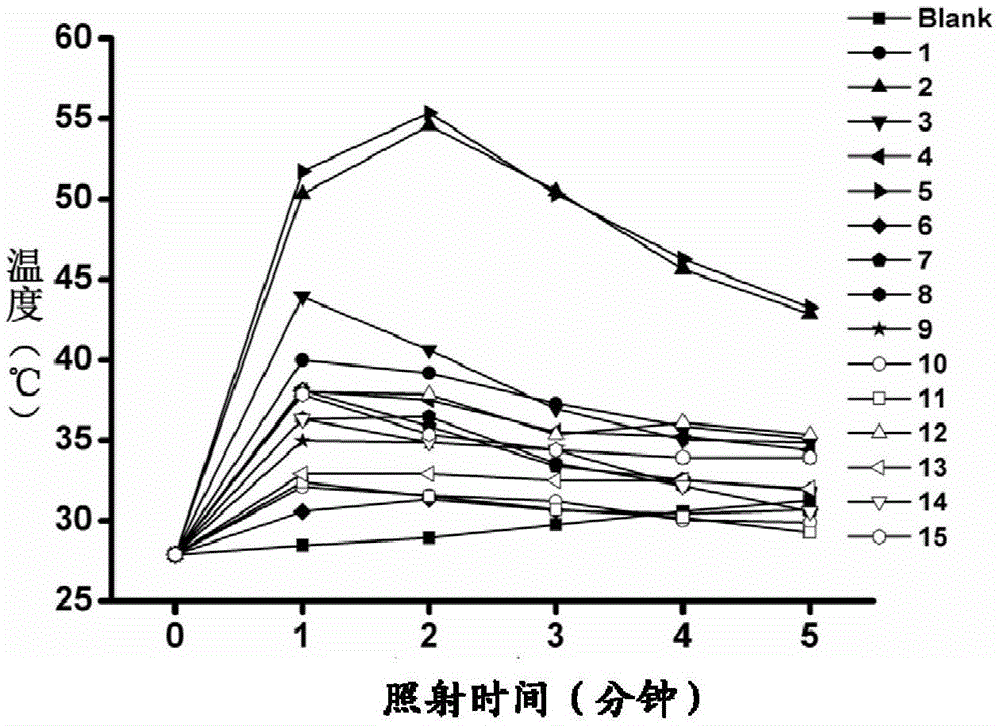
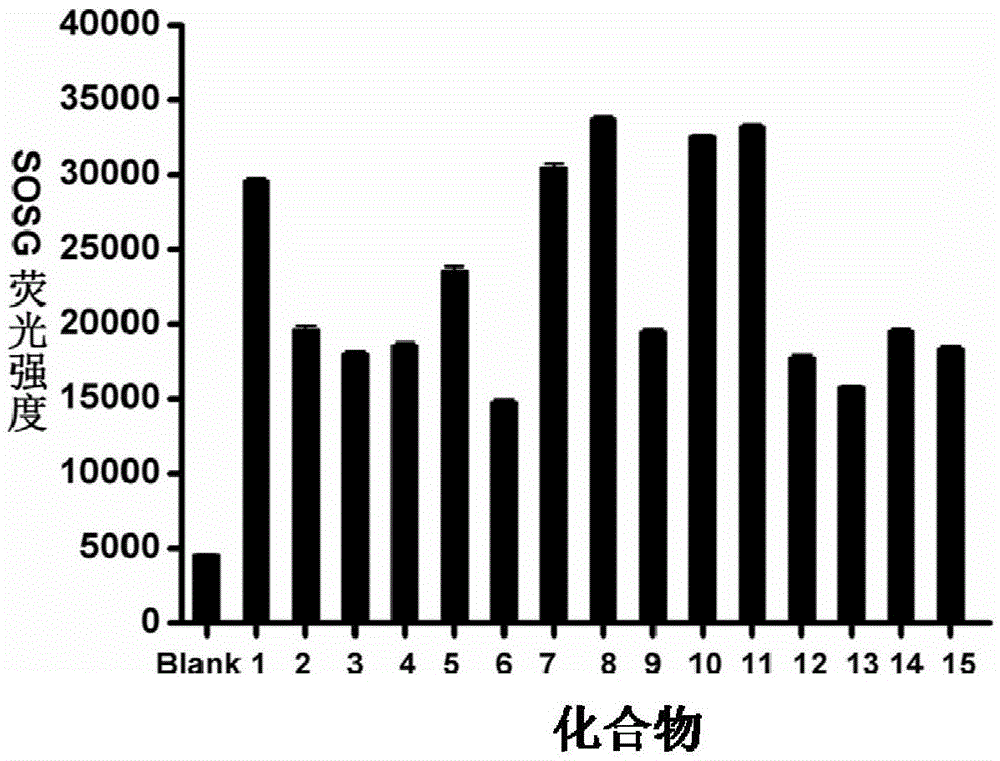
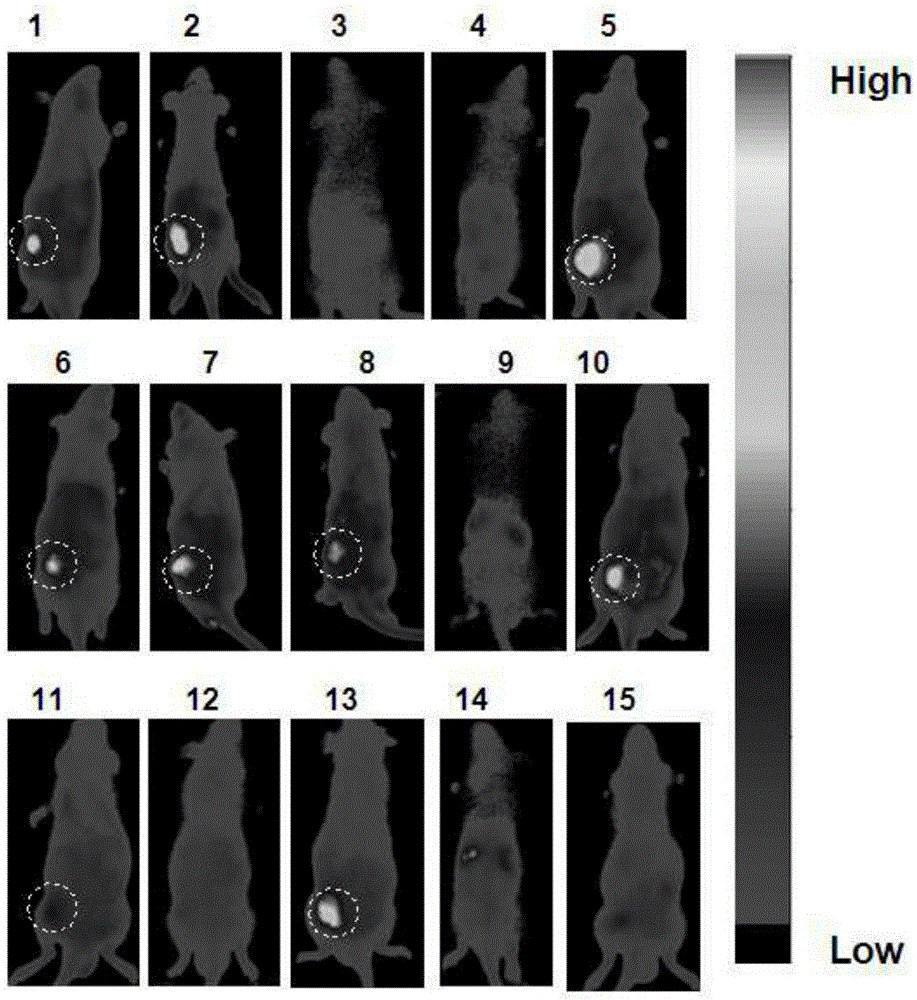
Mitochondrion-targeted heptamethine indocyanine dye, preparation method and application
A technology of heptamethine indole cyanine and mitochondria, applied in the direction of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of Compound A0
[0055] A solution of 35 mL of dry dichloromethane and 37 mL of phosphorus oxychloride was dropped into a mixture of 80 mL of dry dichloromethane and N,N-dimethylformyl (1:1, v / v) under an ice bath. Maintaining at 0°C, 10 g (0.10 mol) of cyclohexanone was added dropwise to the reaction solution, and then the temperature was slowly raised to reflux for 3 h. Stop the reaction, cool in an ice-water bath, and pour the reaction solution into 200 g of crushed ice in batches. A large amount of red solid precipitated out. Filter under reduced pressure, wash the solid in batches with frozen acetone until yellow, and vacuum-dry to obtain a crude product, which is recrystallized from acetone to obtain 12.86 g of a yellow solid (compound A0), yield: 73%, melting point: 130-132°C.
Embodiment 2
[0057] Preparation of Compounds A1-A6:
[0058]
[0059] Take 3.84g (2.41×10 -2 mol)2,3,3-trimethyl-3H-indole in a 100ml single-necked round bottom bottle, add 2.89×10 -2 mol of each brominated organic compound, add 30ml of toluene, react at 110°C for 12h, take a sample and monitor the reaction by TLC (dichloromethane:methanol=15:1), it shows that the reaction of the raw materials is complete, and the toluene is removed by rotary evaporation to obtain a reddish-brown viscous liquid. Roughly recrystallized with dichloromethane-ether to obtain reddish-brown viscous liquids A1-A6. The product was directly subjected to the next reaction without purification.
Embodiment 3
[0061] Preparation of Compound 1-6:
[0062]
[0063] Take 7.75×10 -3 molA1-A6 was added to a 100ml single-mouth round bottom bottle, and 0.67g (3.87×10 -3 mol) A0, add 0.66g (7.75×10 -3 mol) sodium acetate, add 40ml acetic anhydride, stir, react at 70°C for 1h, take a sample TLC to monitor the reaction (dichloromethane:methanol=15:1), it shows that the reaction of raw materials is complete, transfer the reaction solution to a separatory funnel, and use Wash with saturated NaCl solution, anhydrous NaSO 4 Dry, filter, and concentrate under reduced pressure to obtain a reddish-brown liquid, roughly recrystallized with dichloromethane-ether to obtain a reddish-brown solid, and column chromatography to obtain a dark green solid compound 1-6:
[0064] 1 (11%): 1 HNMR (400Hz, DMSO-d 6 )δ: 8.26(d, J=14.4Hz, 2H), 7.94(d, J=8.0Hz, 4H), 7.69(d, J=7.6Hz, 2H), 7.40(t, J=7.2Hz, 8H) ,7.33-7.28(m,2H),6.38(d,J=14.0Hz,2H),5.63(s,4H),2.53-2.50(m,4H),1.73(s,14H); HRMS[M-Br ] + :calc.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com