Graphene composite carbon-coated cobalt-lithium phosphate material and preparation methods and application thereof
A carbon-coated lithium cobalt phosphate and graphene composite technology, applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problem of reducing the energy density of lithium cobalt phosphate batteries, reducing the tap density of lithium cobalt phosphate materials, reducing Active material content and other issues, to achieve the effect of improving electrochemical performance, improving electrochemical performance, and convenient process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh LiF according to the stoichiometric ratio, Co(NO 3 ) 2 ·6H 2 O, H 3 PO 4 Each 0.04mol was dissolved in 500mL water, stirred at 80°C for 2h to obtain a clear solution, and spray-dried at 180°C to obtain cobalt lithium phosphate precursor powder. After the obtained cobalt lithium phosphate precursor is fully mixed with 3% graphene (0.19g) by weight ratio of theoretical cobalt lithium phosphate and sucrose (0.458g) corresponding to 3% carbon residue, ethyl alcohol is used as a solvent for 500r / min ball milling After 6 hours of mixing evenly, the resulting mixture was dried and pressed into tablets, then calcined at 550° C. for 6 hours under the protection of an argon atmosphere, and then cooled to room temperature to obtain a graphene-composite carbon-coated cobalt phosphate material.
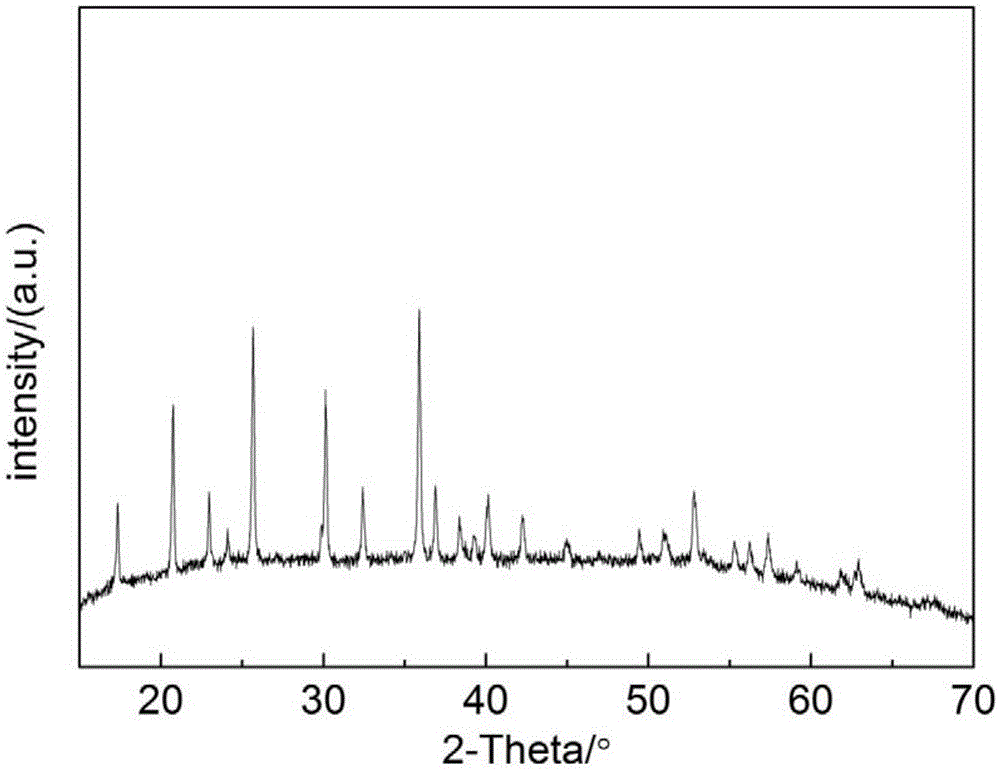
[0032] figure 1 It is the X-ray diffraction (XRD) pattern of the obtained graphene composite carbon-coated cobalt phosphate material. Depend on figure 1 It can be seen that the...
Embodiment 2
[0035] Weigh LiF according to the stoichiometric ratio, Co(NO 3 ) 2 ·6H 2 O, H 3 PO 4 Each 0.04mol and a certain amount of sucrose (0.458g) were dissolved in 300mL of water, stirred at 80°C for 2h to obtain a clear solution (solution A); weigh 3% graphene (0.19g) according to the theoretical weight ratio of lithium cobalt phosphate and disperse it in In 200mL of absolute ethanol, ultrasonically oscillate for a certain period of time until the graphene is uniformly dispersed (solution B); after mixing and stirring solution A and solution B for 60 minutes, spray-dry at 180°C, and the obtained precursor powder is protected under an argon atmosphere Calcined at 550°C for 6 hours, and then cooled to room temperature to obtain a graphene-composite carbon-coated cobalt phosphate material. XRD results show that the prepared lithium cobalt phosphate cathode material has an olivine-type orthorhombic single-phase structure with high phase purity.
Embodiment 3
[0037] Weigh LiNO according to the stoichiometric ratio 3 , Co(NO 3 ) 2 ·6H 2 O, H 3 PO 4 Each 0.04mol was dissolved in 500mL water, stirred at 80°C for 2h to obtain a clear solution, and spray-dried at 180°C to obtain cobalt lithium phosphate precursor powder. After the obtained cobalt lithium phosphate precursor is fully mixed with 3% graphene (0.19g) by weight ratio of theoretical cobalt lithium phosphate and sucrose (0.458g) corresponding to 3% carbon residue, ethyl alcohol is used as a solvent for 500r / min ball milling After 6 hours of mixing evenly, the resulting mixture was dried and pressed into tablets, then calcined at 500° C. for 6 hours under the protection of an argon atmosphere, and then cooled to room temperature to obtain a graphene composite carbon-coated cobalt phosphate material. XRD results show that the prepared lithium cobalt phosphate cathode material has an olivine-type orthorhombic single-phase structure with high phase purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com