Method for synthesizing vinyl acetate
A vinyl acetate, diethylene acetate technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate and other directions, can solve the problems of low vinyl acetate yield, low selectivity, etc., to improve activity and Effects of stability, improved yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
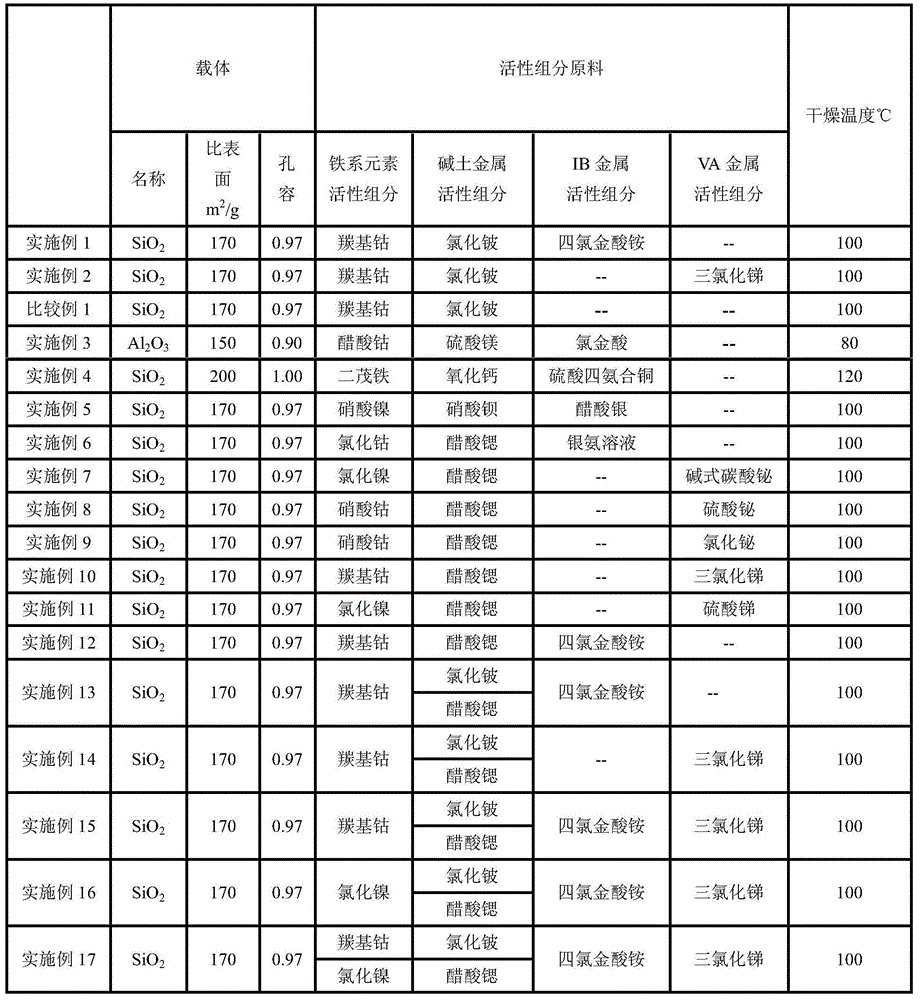
[0027] Preparation of carbonylation catalyst: the Co containing 9.35gCo, containing 3.20gBe and containing 1.85gAu 2 (CO) 8 , BeCl 2 and NH 4 AuCl 4 2H 2 O is fully mixed and dissolved in acetic acid with a concentration of 10wt%, to obtain 400ml of impregnation solution, and the specific surface of 1.0L is 170m 2 / g, spherical SiO with a pore volume of 0.97 and a diameter of 5.6mm 2The carrier was immersed in the above impregnating solution, left to stand for 2 hours and dried at 100° C. to obtain the catalyst. The carbonylation catalyst was analyzed by ICP, and the Co content was 9.35g / L, the Be content was 3.20g / L, and the Au content was 1.85g / L.
[0028] Synthesis of vinyl acetate:
[0029] Step (1): Add 1.5 mol of acetic acid, 0.05 mol of carbonylation catalyst, 0.10 mol of methyl iodide and 0.45 mol of methyl acetate into a 500ml titanium reactor, first use argon to discharge the air in the reactor and pressurize to 2.0MPa, then Introduce carbon monoxide and hydr...
Embodiment 2
[0034] Preparation of carbonylation catalyst: the Co containing 9.35gCo, containing 3.20gBe and containing 1.85gSb 2 (CO) 8 , BeCl 2 and Cl 3 Sb is fully mixed and dissolved in acetic acid with a concentration of 10wt%, to obtain 400ml of impregnation solution, and the specific surface of 1.0L is 170m 2 / g, spherical SiO with a pore volume of 0.97 and a diameter of 5.6mm 2 The carrier was immersed in the above impregnating solution, left to stand for 2 hours and dried at 100° C. to obtain the catalyst. The carbonylation catalyst was analyzed by ICP, and the Co content was 9.35g / L, the Be content was 3.20g / L, and the Sb content was 1.85g / L.
[0035] Synthesis of vinyl acetate:
[0036] Step (1): Add 1.5 mol of acetic acid, 0.05 mol of carbonylation catalyst, 0.10 mol of methyl iodide and 0.45 mol of methyl acetate into a 500ml titanium reactor, first use argon to discharge the air in the reactor and pressurize to 2.0MPa, then Introduce carbon monoxide and hydrogen until t...
Embodiment 3
[0050] Preparation of carbonylation catalyst: Co(OAc) containing 7.00gCo, containing 1.00gMg and containing 1.00gAu 2 4H 2 O, MgSO 4 ·7H 2 O and HAuCl 4 Fully mix and dissolve in pure water to obtain 400ml of impregnation solution, and the specific surface of 1.0L is 150m 2 / g, spherical Al with a pore volume of 0.90 and a diameter of 5.6mm 2 o 3 The carrier was immersed in the above impregnating solution, left to stand for 2 hours and dried at 80° C. to obtain the catalyst. The carbonylation catalyst was analyzed by ICP, and the Co content was 7.00 g / L, the Mg content was 1.00 g / L, and the Au content was 1.00 g / L.
[0051] Synthesis of vinyl acetate:
[0052] Step (1): Add 1.5 mol of acetic acid, 0.05 mol of carbonylation catalyst, 0.10 mol of methyl iodide and 0.45 mol of methyl acetate into a 500ml titanium reactor, first use argon to discharge the air in the reactor and pressurize to 2.0MPa, then Introduce carbon monoxide and hydrogen until the pressure is 8.2MPa, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com