Preparation method of LCZ696 key intermediate
A technology of amino and methyl valerate, which is applied in the field of medicinal chemistry, can solve the problems of industrial production restrictions, expensive commercialization, and difficulty in mass procurement, and achieve the effect of large-scale commercial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
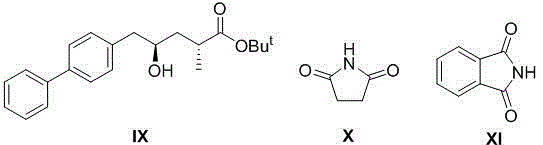
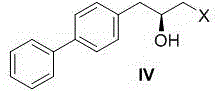
[0026] Example 1: Preparation of (S)-1-([1,1'-biphenyl]-4-yl)-3-chloro-propan-2-alcohol (formula IV, X=Cl)
[0027] In a 500ml three-necked flask, add 5 grams of 4-bromobiphenyl and anhydrous THF (80ml), stir the system evenly and cool to -78°C, then slowly add n-BuLi n-hexane solution (1.6 Minn-Hexane, 15ml). After the dropwise addition, the system was kept at -78°C and stirred for 30 minutes, then slowly raised to -10°C and stirred for 2 hours. A THF solution (3 g, 40 ml THF) of (S)-epichlorohydrin (formula III, X=Cl) was slowly added to the reaction system. After the dropwise addition, the reaction system was naturally warmed up to room temperature and reacted for 5 hours, and then 100 ml of saturated ammonium chloride aqueous solution was slowly added to the reaction system to quench the reaction. Add CH to the system 2 Cl 2 Extract (3×50ml), combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter, remove the solvent under reduced press...
Embodiment 2
[0028] Example 2: Preparation of (S)-1-([1,1'-biphenyl]-4-yl)-3-chloro-propan-2-alcohol (formula IV, X=Cl)
[0029] Add 200 g of 4-bromobiphenyl and anhydrous THF (3.2 L) into a 10 L glass four-neck flask, stir the system evenly and cool to -78°C (dry ice acetone bath), then pour the reaction liquid through the dropping funnel under nitrogen protection. Add n-BuLi in n-hexane solution (1.6 Minn-hexane, 600 mL) slowly. After the dropwise addition, the system was kept at -78°C and stirred for 1 hour, then slowly raised to -10°C and stirred for 4 hours. A THF solution (120 g, 2 L THF) of (S)-epichlorohydrin (Formula III, X=Cl) was slowly added to the reaction system. After the dropwise addition, the reaction system was naturally warmed to room temperature and reacted for 7 hours, and then 2L of saturated ammonium chloride aqueous solution was slowly added to the reaction system to quench the reaction. Add CH to the system 2 Cl 2 Extract (3×2L), combine the organic phases, dry...
Embodiment 3
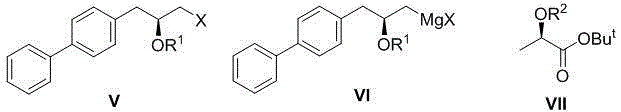
[0030] Example 3: (S)-1-([1,1'-biphenyl]-4-yl)-3-chloro-prop-2-tert-butyldimethylsilyloxy-propane (formula V, R 1 =TBS) preparation
[0031] In a 250mL three-necked round-bottomed flask equipped with a thermometer and magnetic stirring, add (S)-1-([1,1'-biphenyl]-4-yl)-3-chloro-propan-2-ol (formula IV, X=Cl) 8.2 g, then add anhydrous CH 2 Cl2 (80 mL) and DMF (2 mL). After the reaction solution was stirred evenly, DMAP (50mg) was added, and the system was cooled to about 0°C in an ice-water bath. Then slowly add the CH of TBSCl to the reaction system through the dropping funnel 2 Cl 2 solution (6 g, 20 mL CH 2 Cl 2 ), the dropwise addition process kept the temperature of the reaction system lower than 5°C. After the dropwise addition, the system was naturally warmed up to room temperature and stirred for 6 hours. After the reaction was followed by TLC point plate tracking, the solvent was removed under reduced pressure, and the residue was purified by column chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com