Method for purifying semaglutide
A semaglutide and solution technology, which is applied in the field of purification of polypeptide drugs, can solve the problems of difficult production, short service life, cumbersome steps, etc., and achieve the effects of reducing production cost, maintaining biological activity, and improving separation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
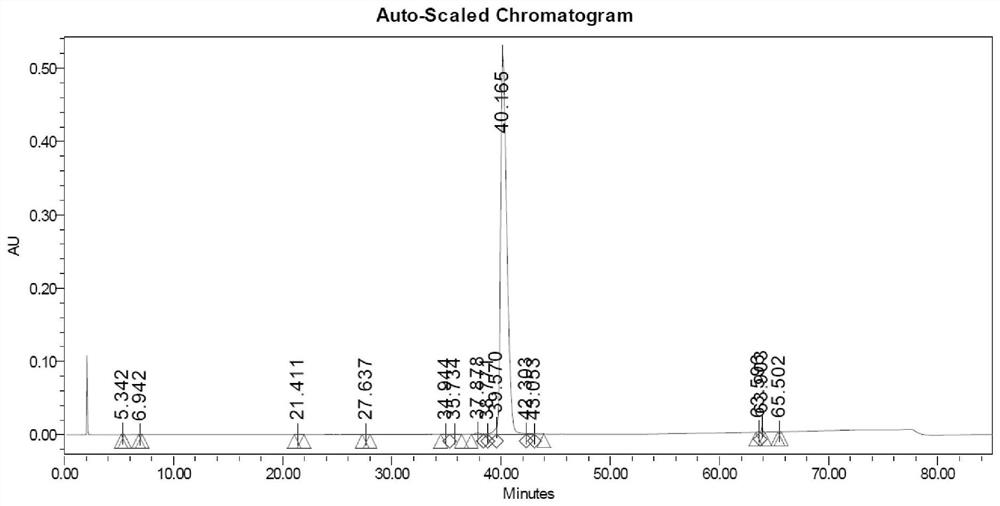
Embodiment 1
[0042] 1. Sample treatment: take 500 grams of crude semaglutide synthesized in solid phase and dissolve it in 5 L of acetic acid aqueous solution (10% concentration by mass) to obtain a crude product solution;
[0043] 2. Precisely filter the crude product solution with a ceramic membrane cross-flow filtration system, and collect the filtrate for later use;
[0044] 3. The first step of reverse phase purification
[0045] Chromatographic conditions: chromatographic column 450X250mm, reversed-phase C8 filler, mobile phase A: 80mMol phosphoric acid solution, adjust pH to 7.2 with triethylamine, mobile phase B: acetonitrile, gradient 40-60%, loading 150 grams, monitoring wavelength The concentration was 230nm, and a linear gradient elution was performed to collect the fraction solution of the first step of semaglutide purification. Use acetic acid to adjust the pH to 6.0, and recover acetonitrile under reduced pressure at 30-32°C to obtain the first-step purification solution fo...
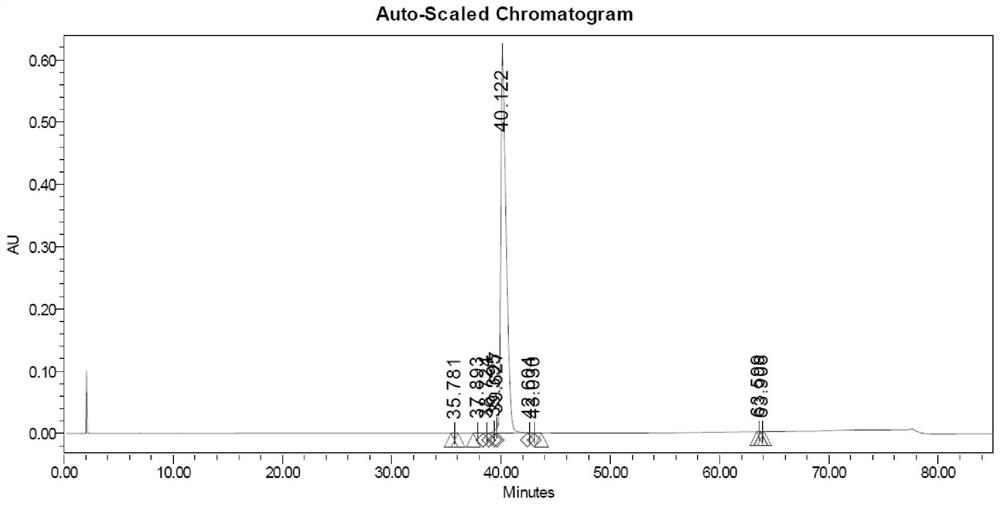
Embodiment 2
[0051]1. Sample treatment: take 800 grams of solid-phase-synthesized crude semaglutide and dissolve it in 8 L of acetic acid aqueous solution (mass percentage concentration 10%) to obtain a crude product solution;
[0052] 2. Precisely filter the crude product solution with a ceramic membrane cross-flow filtration system, and collect the filtrate for later use;
[0053] 3. The first step of reverse phase purification
[0054] Chromatographic conditions: chromatographic column 450X250mm, reversed-phase C8 filler, mobile phase A: 80mMol phosphoric acid solution, adjust pH to 7.2 with triethylamine, mobile phase B: acetonitrile, gradient 40-60%, loading 200 grams, monitoring wavelength The concentration was 230nm, and a linear gradient elution was performed to collect the fraction solution of the first step of semaglutide purification. Use acetic acid to adjust the pH to 5.0, and recover acetonitrile under reduced pressure at 30-32°C to obtain the first-step purification solutio...
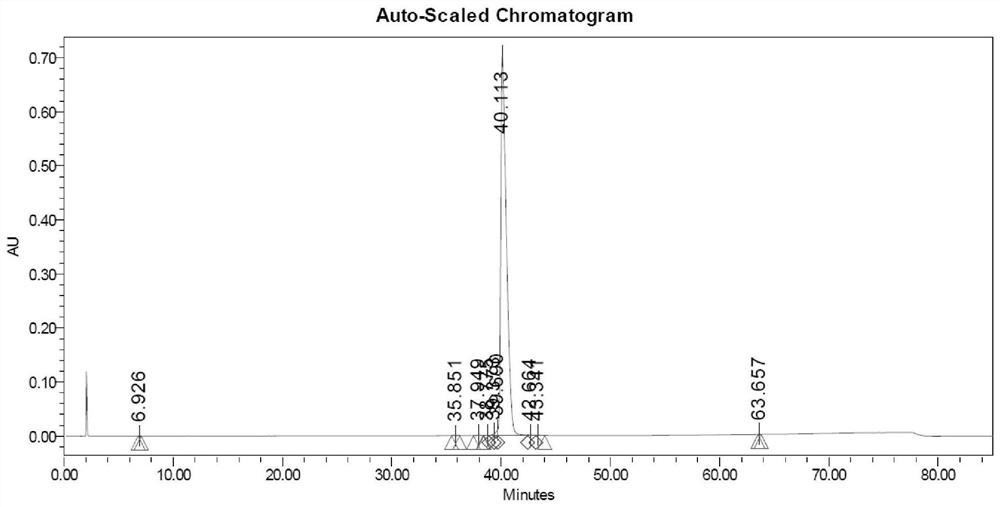
Embodiment 3
[0060] 1. Sample treatment: take 1000 grams of solid-phase synthesized crude semaglutide and dissolve it in 10 L of acetic acid aqueous solution (mass percentage concentration 10%) to obtain a crude product solution;
[0061] 2. Precisely filter the crude product solution with a ceramic membrane cross-flow filtration system, and collect the filtrate for later use;
[0062] 3. The first step of reverse phase purification
[0063] Chromatographic conditions: chromatographic column 450X250mm, reversed-phase C8 filler, mobile phase A: 80mMol phosphoric acid solution, adjust pH to 7.2 with triethylamine, mobile phase B: acetonitrile, gradient 40-60%, loading 250 grams, monitoring wavelength The concentration was 230nm, and a linear gradient elution was performed to collect the fraction solution of the first step of semaglutide purification. Use acetic acid to adjust the pH to 5.5, and recover acetonitrile under reduced pressure at 30-32°C to obtain the first-step purification solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com