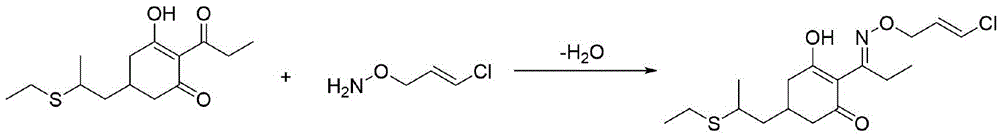Industrialization method for synthesizing O-chloropropene hydroxylamine by virtue of one-pot method
A technology of allylhydroxylamine and dichloropropene, which is applied in the field of industrial synthesis of O-chloroallylhydroxylamine, can solve the problems of increased difficulty in solvent recovery, increased cost, and decreased solvent recovery rate, so as to improve reaction yield and product purity, Reduce the formation of double-connected impurities, avoid the effect of addition and recovery separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 prepares O-chloropropene hydroxylamine with hydroxylamine salt one-pot method:
[0024] Under the environment of feeding nitrogen, drop into ethyl acetate 650kg (molecular weight 88, 7.4kmol), hydroxylamine hydrochloride 500kg (molecular weight 69.5, 7.2kmol), 1kg hexamethylphosphoric triamide in the reactor, stir 0.5 hour, keep The temperature of the reaction solution is between 20-30°C and 1000kg (7.5kmol) of sodium hydroxide solution with a mass concentration of 30% is added dropwise, and after the dropwise addition is completed, the reaction is kept for 1 hour;
[0025] After completion of the reaction, add 1kg Polyethylene Glycol 800 and 1,3-dichloropropene 820kg (molecular weight 111, 7.4kmol) in the reaction solution, add dropwise mass concentration and be 30% sodium hydroxide solution 1000kg, control reaction solution temperature is 30-40°C, stirring for 1.5 hours;
[0026] Add 1000kg (8.2kmol) of hydrochloric acid with a mass concentration of 30% ...
Embodiment 2
[0027] Embodiment 2 prepares O-chloropropene hydroxylamine with hydroxylamine salt one-pot method:
[0028] Under the environment of nitrogen gas, put 810kg of methyl acetate (molecular weight 74, 11.0kmol), 500kg of hydroxylamine hydrochloride (molecular weight 69.5, 7.2kmol), 2kgDMF into the reaction kettle, stir for 0.5 hours, keep at 20-30°C, drop The mass concentration is 1000kg (7.5kmol) of 30% sodium hydroxide solution, and after the dropwise addition is completed, the insulation reaction is carried out for 1 hour;
[0029] After completion of the reaction, add 2 kg of triethylbenzyl ammonium chloride and 970 kg of 1,3-dichloropropene (molecular weight 111, 8.7 kmol) in the reaction solution, and add dropwise a mass concentration of 30% sodium hydroxide solution 1000 kg (7.5 kmol ), and the temperature of the reaction solution is controlled at 30-40°C, and the reaction is stirred for 1 hour;
[0030] Adding 1000kg (8.2kmol) of hydrochloric acid with a mass concentratio...
Embodiment 3
[0031] Embodiment 3 prepares O-chloropropene hydroxylamine with hydroxylamine salt one-pot method:
[0032] Under the environment of nitrogen gas, put 760kg (8.6kmol) of ethyl acetate, 500kg (molecular weight 69.5, 7.2kmol) of hydroxylamine hydrochloride, and 1.5kgDMSO into the reaction kettle, stir for 0.5 hours, keep at 20-30°C, and dropwise add the mass concentration It is 1050kg (7.9kmol) of 30% sodium hydroxide solution, and after the dropwise addition is completed, the insulation reaction is carried out for 1 hour;
[0033] After completion of the reaction, add 1.5kg18-crown 6 and 1,3-dichloropropene 830kg (molecular weight 111, 7.5kmol) to the reaction solution, add dropwise a mass concentration of 30% sodium hydroxide solution 1050kg (7.9kmol), and The temperature of the reaction solution is controlled at 30-40°C, and the reaction is stirred for 1.5 hours;
[0034] Adding mass concentration is 30% hydrochloric acid 1000kg (8.2kmol) in reaction solution, be warming up ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com