Method for degrading chloro-nitroaromatic by using sulfate radicals
A technology of nitroaromatics and free radicals, applied in chemical instruments and methods, water pollutants, water/sludge/sewage treatment, etc., can solve the problems of accelerated reproduction of microorganisms, cost increase, and secondary pollution of the environment, and achieve human and animal Safe, simple method, and non-polluting effect on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
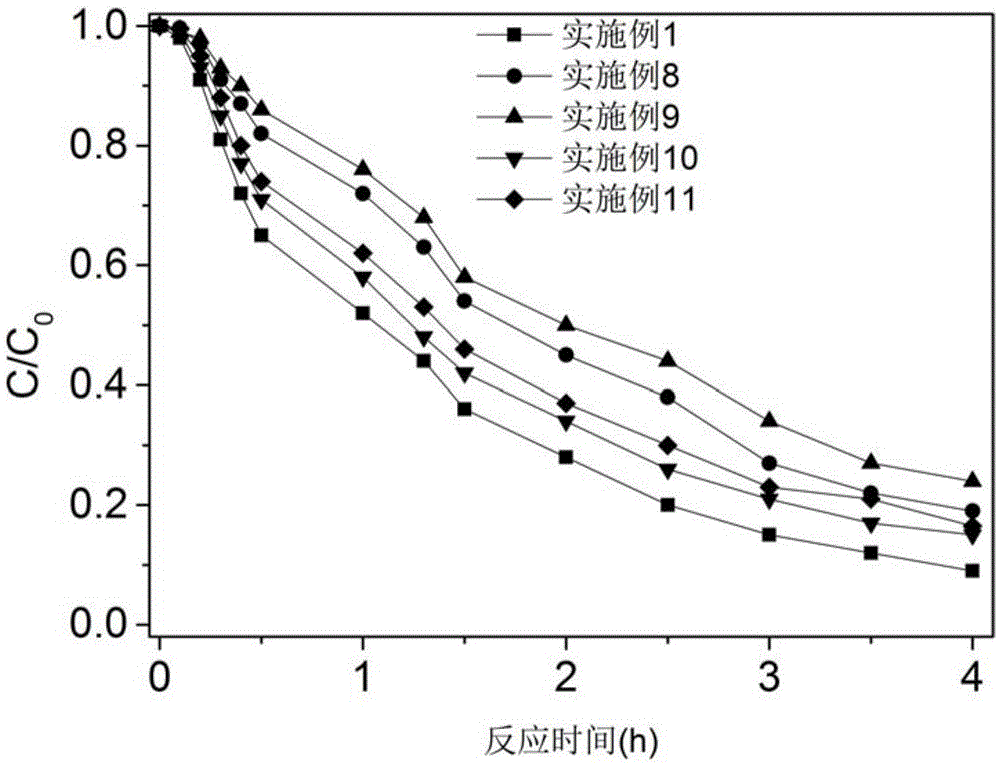
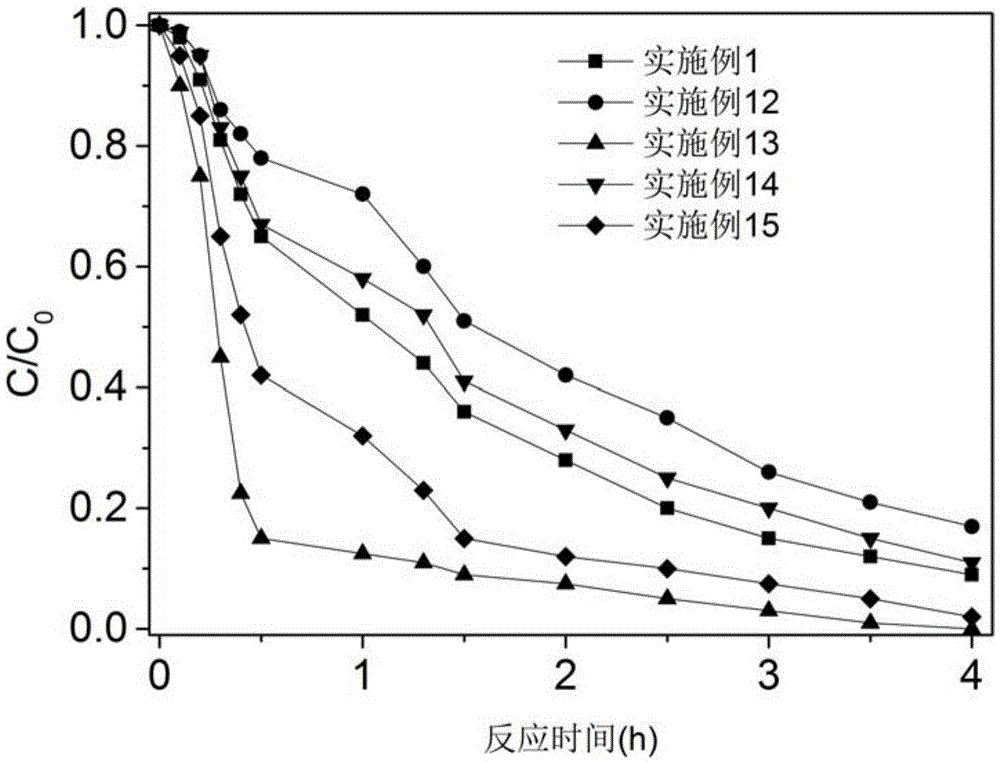
[0027] Take 500ml of water containing 30μM 4-chloronitrobenzene (the concentration of background organic matter is 2.5mg / l, calculated as TOC), and then add 2 millimoles of potassium monopersulfate and 0.06 millimoles of accelerator perillaldehyde to the water. When the concentration of potassium monopersulfate and accelerator perillaldehyde in the solution are 4mM and 0.12mM, respectively, the molar concentration ratio is 1:0.03, and the molar concentration ratio of 4-chlorophenylnitrobenzene and accelerator is 0.25:1) , The pH of the water body is weakly alkaline 7.4, the temperature of the water body is 21 ℃, and then the reaction is uniformly stirred for 4 hours, and the concentration of 4-chloronitrobenzene in the water body is measured every hour during the reaction. The results are as follows figure 1 Shown.
Embodiment 2
[0029] Take 500ml of water containing 10μM 4-chloronitrobenzene (the concentration of background organic matter is 2.5mg / l, calculated as TOC), and then add 2 millimoles of potassium monopersulfate and 0.06 millimoles of accelerator perillaldehyde to the water. When the concentration of potassium monopersulfate and the accelerator perillaldehyde in the solution are 4mM and 0.12mM, respectively, the molar concentration ratio is 1:0.03, and the molar concentration ratio of 4-chlorophenylnitrobenzene and the accelerator is 0.08:1) , The pH of the water body is weakly alkaline 7.4, the temperature of the water body is 21 ℃, and then the reaction is uniformly stirred for 4 hours, and the concentration of 4-chloronitrobenzene in the water body is measured every hour during the reaction. The results are as follows figure 1 Shown.
Embodiment 3
[0031] Take 500ml of water containing 7.5μM 4-chloronitrobenzene (the concentration of background organic matter is 2.5mg / l, calculated as TOC), and then add 2 millimoles of potassium monopersulfate and 0.02 millimoles of accelerator perillaldehyde ( At this time, the concentrations of potassium monopersulfate and the accelerator perillaldehyde in the solution were 4mM and 0.04mM, respectively, and the molar concentration ratio was 1:0.01, and the molar concentration ratio of 4-chlorophenylnitrobenzene and the accelerator was 0.25:1 ), the pH of the water body is weakly alkaline 7.4, the temperature of the water body is 21 ℃, and then the reaction is uniformly stirred for 4 hours, and the concentration of 4-chloronitrobenzene in the water body is measured every hour during the reaction. The results are as follows figure 1 Shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com