Method for continuously preparing letrozole intermediate 4-((1H-1,2,4-tri-1-zole)methyl) benzonitrile
A technology of letrozole and methyl is applied in the field of preparation of letrozole, and can solve the problems of difficulty in product separation, low conversion rate of cyanobenzyl chloride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The qualitative and quantitative detection method of the reaction substrate and product is: using KromasilC 18 Column (12.5cm×4.6mm×5μm), mobile phase: acetonitrile:water (30:70); UV detection wavelength 230nm; flow rate: 1.0mL / min; column temperature 40°C.
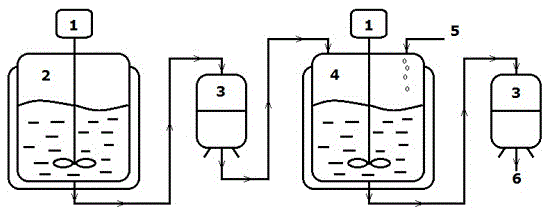
[0019] Pump p-cyanobenzyl chloride, 1,2,4-triazole, potassium carbonate (molar ratio 1:1:1) and solvent acetone into the reaction kettle, heat to the reaction temperature of 75°C, and stir vigorously for 3 hours. The reaction solution is pumped into the suction filter tank from the bottom of the reaction kettle, and the filtrate flows out from the bottom of the tank into the crystallization kettle, and 15% concentrated hydrochloric acid is added to carry out salt formation and crystallization for 2 hours, and then pumped into the suction filter tank, the obtained reaction product 4-((1H The yield of -1,2,4-tris-1-azole)methyl)benzonitrile is greater than 82.61%, and the chemical purity is greater than 98.75%.
Embodiment 2
[0021] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0022] Pump p-cyanobenzyl chloride, 1,2,4-triazole, potassium carbonate (molar ratio 1:3:1) and solvent acetone into the reaction kettle, heat to the reaction temperature of 70°C, and vigorously stir for 3.5 hours. Pump the reaction liquid from the bottom of the reaction kettle into the suction filter tank, the filtrate flows out from the bottom of the tank into the crystallization kettle, add 17% concentrated hydrochloric acid to carry out salt formation, salt crystallization and crystallization for 3 hours, and then pump into the suction filter tank, the obtained reaction product 4- The yield of ((1H-1,2,4-tri-1-azole)methyl)benzonitrile was greater than 83.61%, and the chemical purity was greater than 98.88%.
Embodiment 3
[0024] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0025] Pump p-cyanobenzyl chloride, 1,2,4-triazole, potassium carbonate (molar ratio 1:5:1) and solvent acetone into the reaction kettle, heat to the reaction temperature of 60°C, and stir vigorously for 5 hours. The reaction solution is pumped into the suction filter tank from the bottom of the reaction kettle, and the filtrate flows out from the bottom of the tank into the crystallization kettle, and 18% concentrated hydrochloric acid is added to carry out salt formation, salt crystallization and crystallization for 1h, and then pumped into the suction filter tank, and the obtained reaction product 4-( The yield of (1H-1,2,4-tri-1-azole)methyl)benzonitrile was greater than 84.51%, and the chemical purity was greater than 98.98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com