Improved separation preparation method for mouse thoracic aorta vascular smooth muscle cells
A technology for vascular smooth muscle and thoracic aorta, which is applied in the fields of biology and medicine, can solve the problem of smooth muscle cell damage in the membrane layer, and achieve the effect of shortening the digestion time, avoiding the adventitia residue, and stabilizing the digestion time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Separation test of primary rat thoracic aorta VSMC:
[0033] Compound enzyme liquid formula: type II collagenase 1mg / ml (Worthington Biochemical Corporation, 44N15309A, USA), trypsin inhibitor 1mg / ml (Worthington Biochemical Corporation, R4H15000, USA), trypsin 7.08μl / ml (Worthington Biochemical Corporation, 35M16094, USA), with Ca 2+ , Mg 2+ HBSS was used as the solvent, and the fixed volume was 1ml; each rat thoracic aorta was digested with an average of 1ml compound enzyme each time.
[0034] The rat thoracic aorta used in this example is a healthy SPF SD rat with a body weight of 90-120 g. Take ether to anesthetize the animal, transfer to the sterile operating table, and fix the animal's abdominal cavity upward. First spray the rat’s chest and abdomen with 75% alcohol, cut open the abdomen and chest cavity in turn, take a cotton swab to remove the internal organs, and expose the thoracic aorta blood vessels; then use ophthalmic scissors to carefully rem...
Embodiment 2
[0039] Embodiment 2 Culture test of primary rat thoracic aortic smooth muscle:
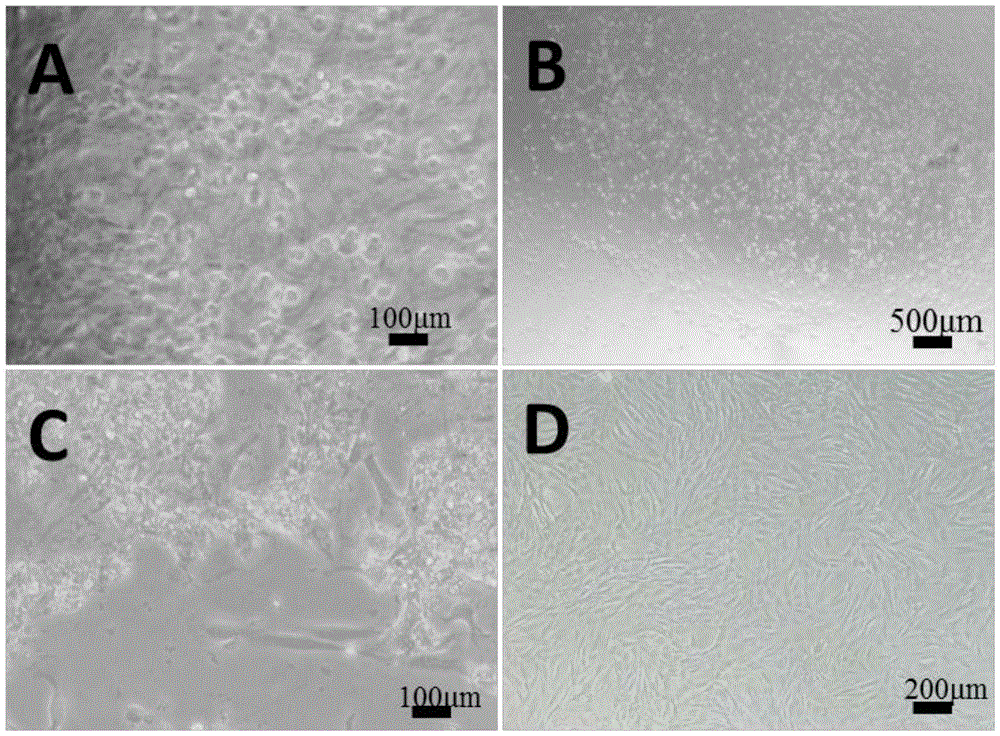
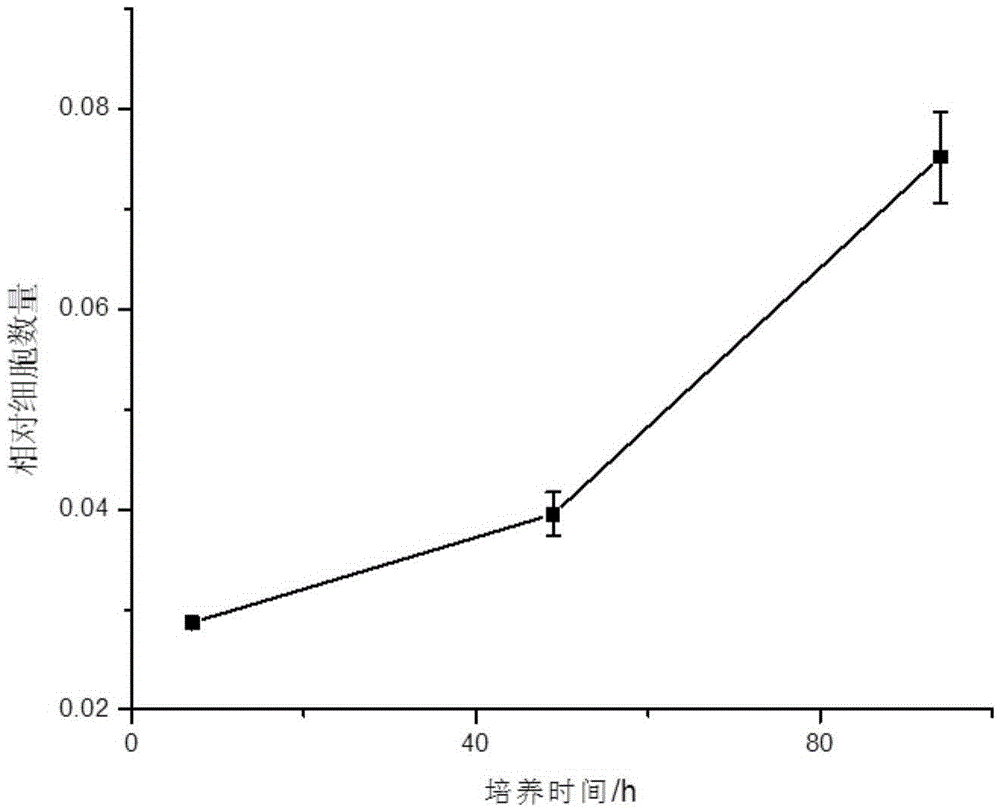
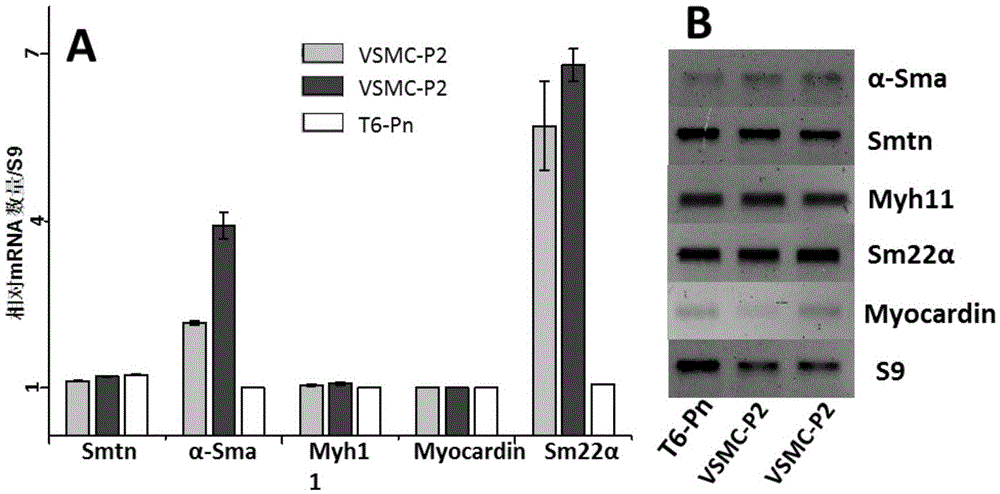
[0040] Freshly isolated P0 generation VSMCs were suspended in DMEM containing 20% FBS and 1% double antibody, and cultured statically in an incubator for 2 days at 37°C, 5% CO 2 . Observed after 48 hours, it was found that the cells had adhered to the wall and started to spread; replaced with a new DMEM medium containing 20% FBS and 1% double antibody and then cultured for about 7 days. In one 10cm culture dish (generation P1); about 7 days later, the cells of generation P1 can cover the culture dish, and passage to 2-3 culture dishes of 10cm (generation P2). After the P2 generation cells are congested, cryopreservation begins. P0-2 generation VSMC were cultured in DMEM containing 20% FBS and 1% double antibody, and VSMC after P3 generation were cultured in F-12 / DMEM (1:1) containing 10% FBS and 1% double antibody.
Embodiment 3
[0041] Embodiment 3 Passaging of primary rat thoracic aortic smooth muscle:
[0042] When the confluence of VSMC reaches 80-90%, it can be subcultured. Taking P0 generation VSMC as an example, aspirate the culture medium in the 6-well plate, add 1ml PBS solution to rinse the cells to remove residual serum and dead cells. Add an appropriate amount of 0.25% trypsin solution, shake gently, and place in a 37°C incubator to digest for about 10s-20s. It can be observed under an inverted phase-contrast microscope that the cells begin to shrink into a round shape, and the connections between cells obviously disappear and appear. void. At this point, serum-containing medium was added to terminate trypsinization. Use a pipette to blow and blow the cells gently to make a single-cell suspension, and observe the blowing and blowing of the cells under an inverted phase-contrast microscope. Transfer the single cell suspension into a 15ml centrifuge tube and centrifuge at 1000 rpm for 5 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com