Tungsten carbide shell layer coated lithium iron phosphate positive electrode material and preparation method therefor
A technology of lithium iron phosphate and positive electrode materials, which is applied in battery electrodes, electrochemical generators, electrical components, etc., can solve the problems of lower volume specific capacity and unsatisfactory lithium iron phosphate, and achieve excellent rate performance and good binding force Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
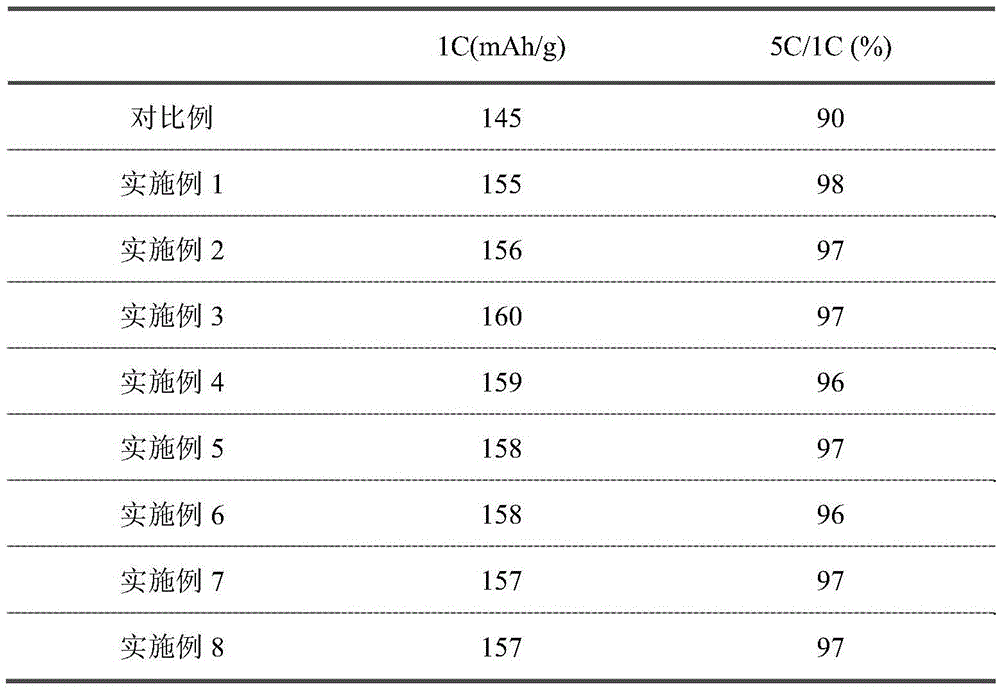
[0020] Weigh 0.1 mol of ferrous sulfate, 0.11 mol of lithium chloride and 0.1 mol of ammonium dihydrogen phosphate, respectively dissolve them in 200ml of deionized water, mix them together and pour them into a hydrothermal reaction tank, add ammonia water, control the pH to 9.0, and seal Placed in a heater, 200 ℃ hydrothermal reaction for 24h. After cooling, the precipitate was repeatedly centrifuged and washed with deionized water, and the separated powder was vacuum-dried at 100°C for 10 hours to obtain lithium iron phosphate (LiFePO 4 sample). The LiFePO 4 The sample was placed in the reaction chamber of the plasma-enhanced chemical vapor deposition equipment, and the vacuum was evacuated to 1.0×10 -1 Pa, and heated to 300°C. Charge WF 6 +CH 4 (WF 6 :CH 4 =1:1 volume ratio) mixed gas, the total pressure of the reaction chamber was maintained at 50Pa. The power output of the RF plasma is 600W. The reaction time was controlled at 25min. A lithium iron phosphate pos...
Embodiment 2
[0022] Weigh 0.1 mol of ferrous sulfate, 0.11 mol of lithium chloride and 0.1 mol of ammonium dihydrogen phosphate, respectively dissolve them in 200ml of deionized water, mix them together and pour them into a hydrothermal reaction tank, add ammonia water, control the pH to 9.0, and seal Placed in a heater, 250 ℃ hydrothermal reaction for 15h. After cooling, the precipitate was repeatedly centrifuged and washed with deionized water, and the separated powder was vacuum-dried at 100°C for 18 hours to obtain lithium iron phosphate (LiFePO 4 sample). The LiFePO 4 The sample was placed in the reaction chamber of the plasma-enhanced chemical vapor deposition equipment, and the vacuum was evacuated to 1.0×10 -1 Pa, and heated to 400°C. Charge WF 6 +CH 4 (WF 6 :CH 4 =1:1.5 volume ratio) mixed gas, the total pressure of the reaction chamber is maintained at 10Pa. The power output of the RF plasma is 800W. The reaction time was controlled within 10 minutes. A lithium iron pho...
Embodiment 3
[0024] Weigh 0.1 mol of ferrous sulfate, 0.11 mol of lithium chloride and 0.1 mol of ammonium dihydrogen phosphate, respectively dissolve them in 200ml of deionized water, mix them together and pour them into a hydrothermal reaction tank, add ammonia water, control the pH to 9.0, and seal Placed in a heater, 300 ℃ hydrothermal reaction 10h. After cooling, the precipitate was repeatedly centrifuged and washed with deionized water, and the separated powder was vacuum-dried at 100°C for 17 hours to obtain lithium iron phosphate (LiFePO 4 sample). The LiFePO 4 The sample was placed in the reaction chamber of the plasma-enhanced chemical vapor deposition equipment, and the vacuum was evacuated to 1.0×10 -1 Pa, and heated to 500°C. Charge WF 6 +CH 4 (WF 6 :CH 4 =1:1.2 volume ratio) mixed gas, the total pressure of the reaction chamber is maintained at 100Pa. The power output of the RF plasma is 400W. The reaction time was controlled at 60min. A lithium iron phosphate posit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com