Diacid monomer containing diphenylamine-fluorene, preparation method and application thereof for preparing polyamide
A technology of diacid monomers and diamine monomers, which is applied in the field of preparing polyamides with electrochromic properties, which can solve the problems of few varieties of polyamides, achieve the effects of preventing accumulation, maintaining thermal performance, and increasing free volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
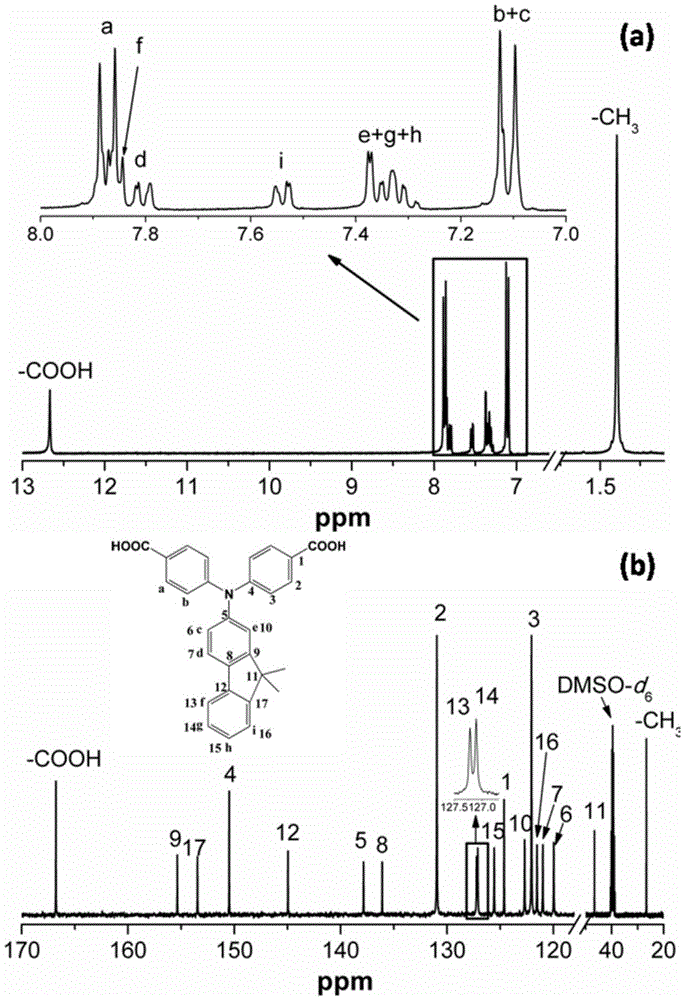
[0037] Example 1: Preparation of N,N-bis(4-carboxyphenyl)-9,9-dimethylfluorene
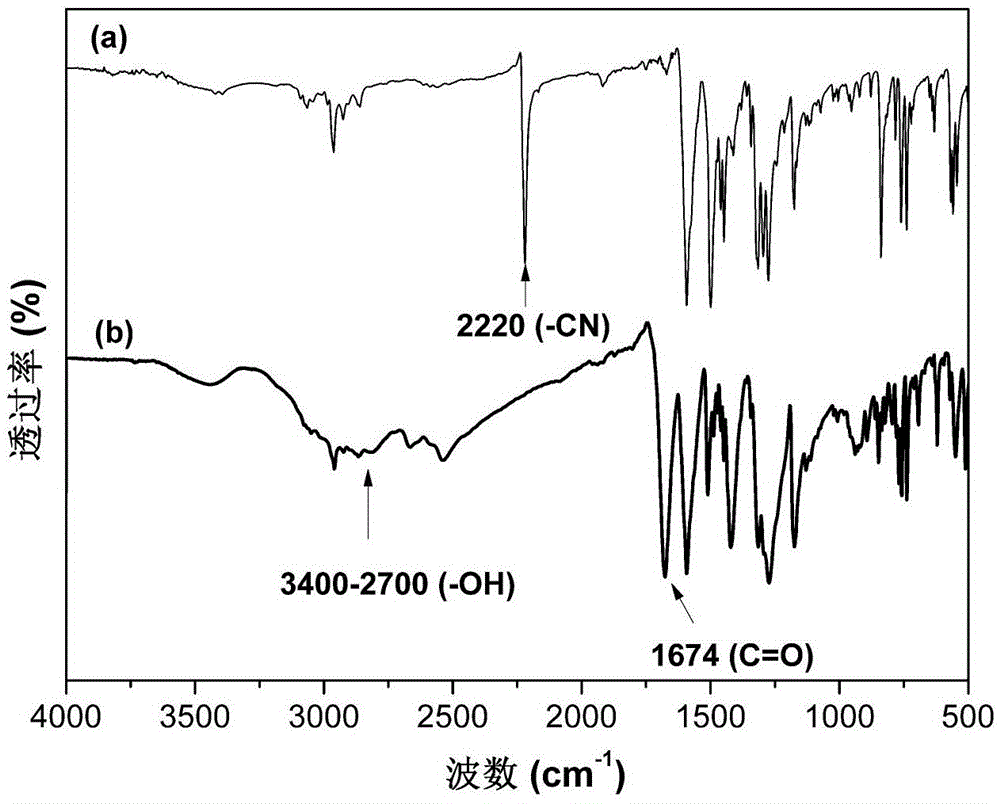
[0038] The first step reaction: 10.0 g (48 mmol) of 2-amino-9,9-dimethylfluorene, 11.6 g (96 mmol) of p-fluorobenzonitrile, 14.6 g ( 96 mmol) of cesium fluoride, 100 mL of dimethyl sulfoxide was added as a solvent, and the reaction was carried out at 170° C. for 18 h under stirring and nitrogen protection. After cooling, the material was discharged into ice water, the crude product was washed twice with water, and recrystallized with glacial acetic acid to obtain 13.2 g of dark yellow N,N-bis(4-cyanophenyl)-9,9-dimethylfluorene crystals , the yield is 67%.
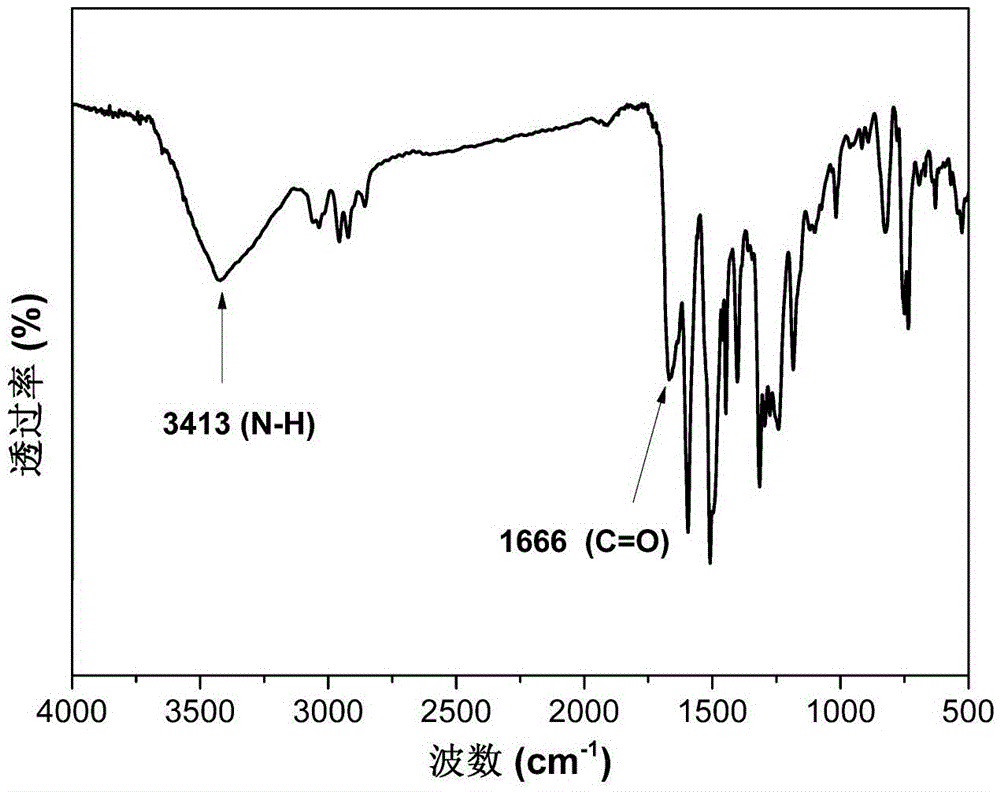
[0039]The second step reaction: Add 8 g of N,N-bis(4-cyanophenyl)-9,9-dimethylfluorene prepared in the first step into a 100mL three-necked flask equipped with a magnetic stirrer and a condenser powder, 16.0g potassium hydroxide, add 20mL ethanol and 20mL water as solvent, heat to reflux, continue to reflux and stir for 72h. After cooling, ...
Embodiment 2
[0042] Example 2: Preparation of N,N-bis(4-carboxyphenyl)-9,9-diphenylfluorene
[0043] The first step reaction: add 15.0g (45mmol) of 2-amino-9,9-diphenylfluorene, 10.9g (90mmol) of p-fluorobenzonitrile, 13.7g ( 90mmol) of cesium fluoride, add 120mL of dimethyl sulfoxide as a solvent, and react for 26h at 170°C under stirring and nitrogen protection. After cooling, discharge the material into ice water, wash the crude product twice with water, and recrystallize with glacial acetic acid to obtain 15.2 g of dark yellow N,N-bis(4-cyanophenyl)-9,9-diphenylfluorene powder , yield 63%.
[0044] The second step reaction: Add 10 g of N,N-bis(4-cyanophenyl)-9,9-diphenylfluorene prepared in the first step into a 100mL three-necked flask equipped with a magnetic stirrer and a condenser Powder, 15.7g potassium hydroxide, add 25mL ethanol and 15mL water as solvent, heat to reflux, continue to reflux and stir for 72h. After cooling, use concentrated hydrochloric acid (6mol / L) to adjust ...
Embodiment 3
[0046] Example 3: Preparation of N,N-bis(4-carboxyphenyl)-9,9-dihexylfluorene
[0047] The first step reaction: add 15.0g (43mmol) of 2-amino-9,9-dihexylfluorene, 10.4g (86mmol) of p-fluorobenzonitrile, 13.1g (86mmol) in a 250mL three-necked flask equipped with mechanical stirring ) of cesium fluoride, add 120 mL of dimethyl sulfoxide as a solvent, and react for 26 hours at 170° C. under stirring and nitrogen protection. After cooling, discharge the material into ice water, wash the crude product twice with water, and recrystallize from glacial acetic acid to obtain 14.4 g of dark yellow N,N-bis(4-cyanophenyl)-9,9-dihexylfluorene powder. Yield 61%.
[0048] The second step reaction: Add 10 g of N,N-bis(4-cyanophenyl)-9,9-dihexylfluorene powder prepared in the first step into a 100mL three-necked flask equipped with a magnetic stirrer and a condenser 15.0g potassium hydroxide, add 25mL ethanol and 15mL water as a solvent, heat to reflux, continue to reflux and stir for 72h. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com