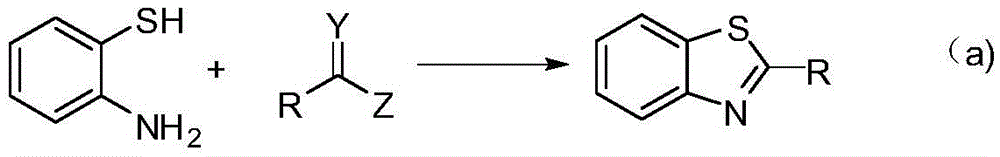
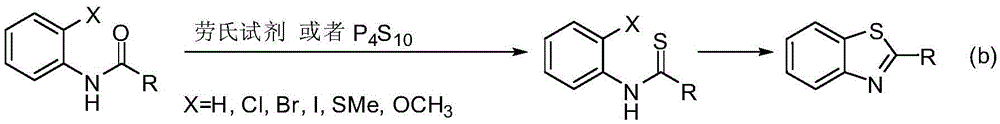
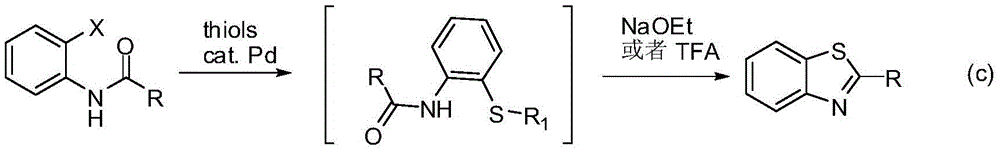
Method for synthesizing benzothiazole derivatives
A synthetic method, benzothiazole technology, applied in the field of organic synthesis, can solve the problems of uneconomical and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Add N-oxalyl-substituted 2-(4-methoxy-benzylthio)-4-bromo-aniline (0.2mmol), trifluoroacetic acid (0.1ml) and thiourea to a 50ml sealed tube. (0.2mmol). The reaction was heated at 80°C, and the reaction was detected by TLC. After the reaction was completed, saturated sodium bicarbonate solution was added to quench the reaction. It was extracted three times with 30 ml of n-hexane, and the upper organic phase was evaporated to dryness to obtain 6-bromo-2-ethyl acetate benzothiazole with a yield of 95%. Then add 1mol / L sodium hydroxide solution (1ml), 4-bromo-2-fluoro-nitrobenzene (0.2mmol) to the lower aqueous phase, stir the reaction at room temperature, check the reaction by TLC, add 15ml after the reaction is complete Extraction with ethyl acetate, dry with anhydrous sodium sulfate, rotary evaporate the organic phase, and column chromatography (petroleum ether: ethyl acetate = 7:1) to obtain 4-bromo-2-(4-methoxy-benzylthio) -Nitrobenzene, the yield is 80%.
[0046] ...
Embodiment 2
[0049] 1. Add N-oxalyl substituted 2-(2-methoxy-benzylthio)-aniline (0.2mmol), trifluoroacetic acid (0.1ml) and thiourea (0.2mmol) to a 50ml sealed tube. . The reaction was heated at 80°C, and the reaction was detected by TLC. After the reaction was completed, saturated sodium bicarbonate solution was added to quench the reaction. It was extracted three times with 30 ml of n-hexane, and the upper organic phase was evaporated to dryness to obtain 2-ethyl acetate benzothiazole with a yield of 95%. Then add 1mol / L sodium hydroxide solution (1ml), 2-fluoro-nitrobenzene (0.2mmol) to the lower aqueous phase, stir the reaction at room temperature, TLC check the reaction, add 15ml of ethyl acetate after the reaction is complete Extraction, drying with anhydrous sodium sulfate, rotary evaporation of the organic phase, and column chromatography (petroleum ether: ethyl acetate = 7:1) to obtain 2-(2-methoxy-benzylthio)-nitrobenzene with a yield 78%.
[0050] 2. Add 2-(2-methoxy-benzylthio...
Embodiment 3
[0053] 1. Add N-benzoyl substituted 2-(4-methoxy-benzylthio)-6-methoxy-aniline (0.2mmol) and trifluoroacetic acid (0.1ml) to a 50ml sealed tube. And thiourea (0.2 mmol). The reaction was heated at 80°C, and the reaction was detected by TLC. After the reaction was completed, saturated sodium bicarbonate solution was added to quench the reaction. It was extracted three times with 30ml of n-hexane, and the upper organic phase was evaporated to dryness to obtain 4-bromo-2-phenylbenzothiazole with a yield of 95%. Then add 1mol / L sodium hydroxide solution (1ml), 6-methoxy-2-fluoro-nitrobenzene (0.2mmol) to the lower aqueous phase, stir the reaction at room temperature, check the reaction by TLC, and after the reaction is complete Add 15ml of ethyl acetate for extraction, dry with anhydrous sodium sulfate, evaporate the organic phase, and column chromatography (petroleum ether: ethyl acetate = 7:1) to obtain 6-methoxy-2-(4-methoxy- Benzylthio)-nitrobenzene, the yield was 82%.
[0054...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com