Purification method of pseudomonas aeruginosa vaccine recombinant protein Vac11
A technology of Pseudomonas aeruginosa and purification method, which is applied in the field of separation and purification of Pseudomonas aeruginosa recombinant protein Vac11, which can solve the problems of reported and unseen purification methods of recombinant protein Vac11
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
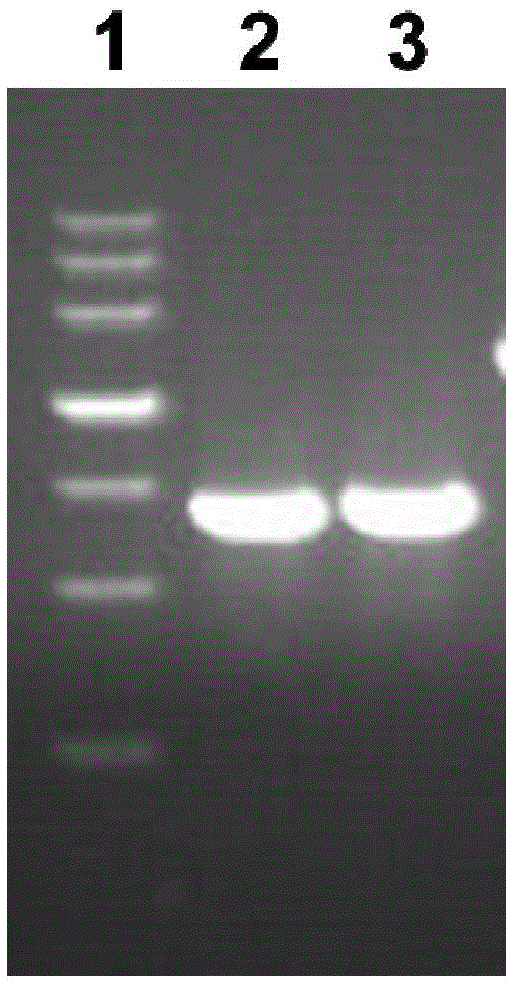
[0066] Example 1 Cloning of Vac11 Gene and Construction of Recombinant Plasmid pGEX-6P-2-Vac11
[0067] 1. According to the full-length gene sequence of AmpDh3 protein of Pseudomonas aeruginosa PA01, the bioinformatics software was used for structural analysis to determine the target gene fragment of Vac11 to be amplified.
[0068] 2. According to the analysis results, the PCR method was used to amplify the AmpDh3 target gene fragment from the PA01 genome, and the amplification steps were as follows:
[0069] 1) Design the PCR primers as follows, which are SEQ ID NO: 3-4 (the base sequence of the restriction site is underlined)
[0070] Seq ID
Primer name
Sequence (5'-3')
Seq ID 3
Vac11-F
ATCGGATCCATGCTGACCATCGACT
Bam H I
Seq ID 4
Vac11-R
CCGGAATTCTCAGGCCGGGTATTTC
EcoR I
[0071] 2) Take out the preserved Pseudomonas aeruginosa strain PA01 from the freezer at -80°C and spread it on LB solid medi...
Embodiment 2
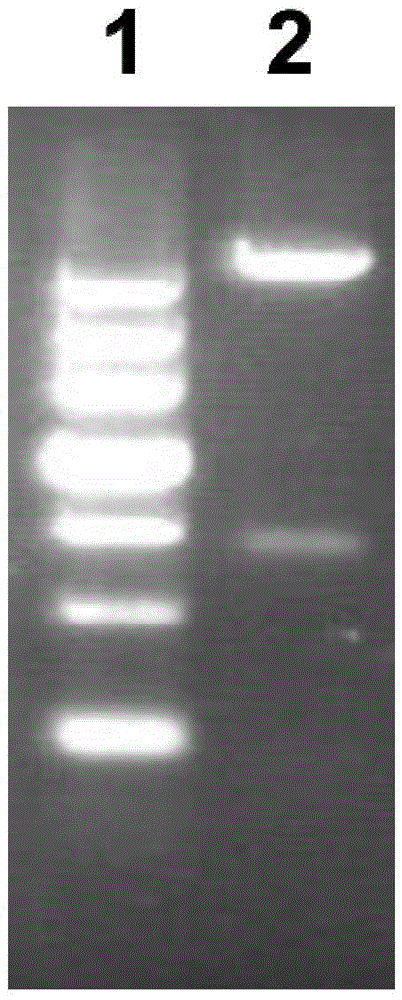
[0101] Example 2: Induced expression of recombinant fusion protein Vac11 in prokaryotic expression system-Escherichia coli and identification of its expression form
[0102] 1. Vac11 induced expression
[0103] Take 100 μL of the overnight cultured pGEX-6P-2-Vac11 / XL-1blue bacterial solution and add it to 10 mL of Amp+ resistant LB medium, culture overnight at 180 rpm at 37°C, take 400 μL of the overnight cultured bacterial solution and add it to 20 mL of Amp+ resistant LB medium (The rest of the bacterial solution is stored in a refrigerator at 4°C for later use), culture at 37°C for 2-3 hours, rotate at 200 rpm, and reactivate until the OD600 is 0.8-1.0, add 4 μL of IPTG to make the final concentration 200 μM, and place on a shaker Induced expression Induced expression at 30°C for 3h.
[0104] 2) Take out the bacterial solution after induced expression, centrifuge at 12000rpm for 5min, discard the supernatant, add 1mLlysisbuffer (20mMPB, pH7.2, 250mMNacl) to mix, ultrasonic...
Embodiment 3
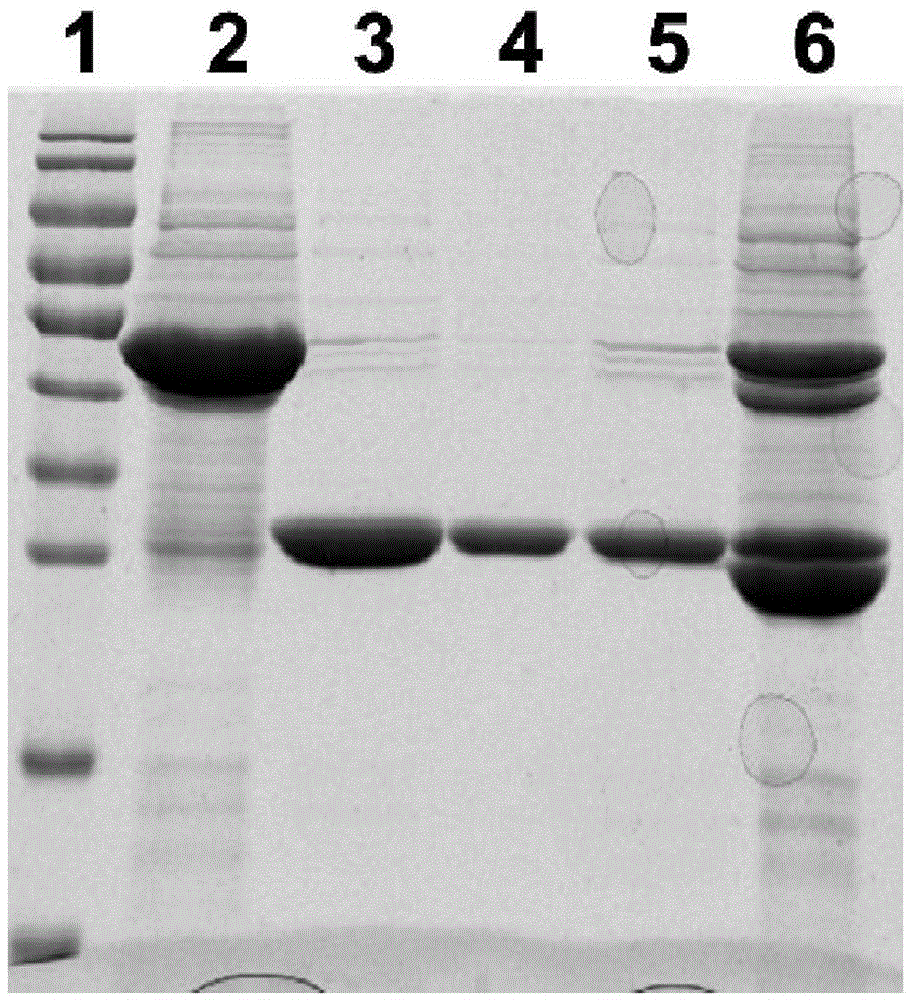
[0111] Embodiment 3: Preparation of Vac11 antigen
[0112] 1. Amplify culture to obtain protein
[0113] Take 400 μL of the spare pGEX-6P-2-Vac11 / XL-1blue bacterial solution stored in the 4°C refrigerator and add it to 20mL LB medium containing Amp resistance for one activation. After culturing at 200rpm for 5-6 hours at 37°C, take 8mL once The activated bacterial solution was added to 400mL LB medium containing Amp resistance for secondary activation, cultured at 37°C for 3-4h until the OD600 was 1.0, then added 80μLPTG (final concentration 200μM) and placed in a shaker at 16°C overnight to induce Finally, 12000rpm was centrifuged for 15min to collect the thalli, and after adding 20mLlysisbuffer (same as Example 2) to resuspend the thallus, the bacterium liquid was subjected to ultrasonic cracking for 3min (200V), and the supernatant was collected and 800 μL of GlutathioneSepharose4B (GE Company) gel beads (beads) binding treatment; then SDS-PAGE gel electrophoresis.
[011...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com