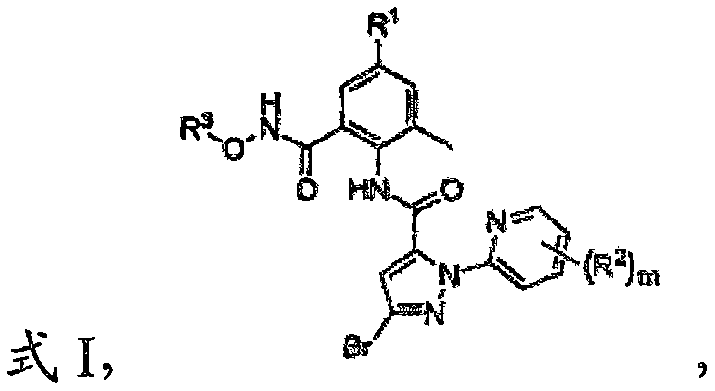
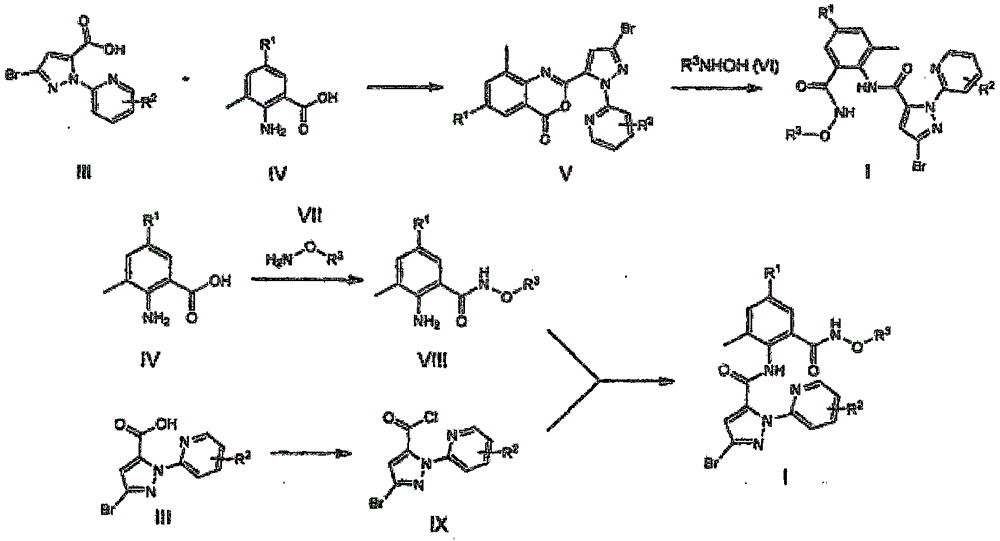
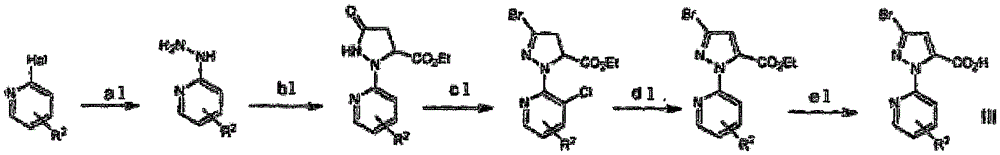
O-carboxamido benzamide derivative based on ryanodine receptor, and preparation method and application thereof
A technology for o-formamidobenzamide and formamidobenzamide, which is applied in the field of o-formamidobenzamide derivatives and the preparation thereof, and can solve problems such as limited number of varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: 3-bromo-1-(3-chloropyridin-2-yl)-N-(4-cyano-2-(methoxycarbamoyl)-6-methylphenyl)-1H- Preparation of pyrazole-5-carboxamide (compound I-10)
[0068]
[0069] Compound I-10 can be prepared by the following method, and the specific steps are as follows:
[0070]
[0071] Preparation of intermediate 3-bromo-1-(3-chloro-pyridyl)-1H-pyrazole-5-carboxylic acid III-1:
[0072]
[0073] Preparation of 2-(3-bromo-pyrazol-1-yl)-3-chloropyridine:
[0074] 3-Bromo-1H-pyrazole was dissolved in DMF (80 mL), 2,3-dichloro-pyridine (13.8 g) and potassium carbonate (57.3 g) were added, and the mixture was stirred at 100° C. for 8 hrs. After adding water, the mixture was extracted twice with ethylamine, the combined organic layers were washed twice with water, brine, washed with Na 2 SO 4 Dry and concentrate in vacuo. The residue was purified by column chromatography (propylamine / ethylamine 6:1) to give 7 g of the title compound 2-(3-bromo-pyrazol-1-yl)-3-chloropyr...
Embodiment 2
[0082] Example 2: 3-bromo-N-(4-chloro-2-methyl-6-(carboxycarbamoyl)phenyl)-1-(3,5-dichloropyridin-2-yl)-1H- Preparation of pyrazole-5-carboxamide (compound 1-2)
[0083]
[0084] Compound I-2 can be prepared by the following method, and the specific steps are as follows:
[0085] Preparation of 2-(3-bromo-1H-pyrazol-1-yl)-3,5-dichloropyridine:
[0086]
[0087] Compound I-2 can be prepared by the following method, and the specific steps are as follows:
[0088] Preparation of 2-(3-bromo-1H-pyrazol-1-yl)-3,5-dichloropyridine:
[0089]
[0090] This compound is carried out in the same manner as for the preparation of 2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine, from 3-bromo-1H-pyrazole and 2,3,5-trichloropyridine to prepare.
[0091] Preparation of 3-bromo-1-(3,5-dichloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid (III-2):
[0092]
[0093] Compound III-2 was prepared from 2-(3-bromo-1H-pyrazol-1-yl)-3,5-dichloropyridine and carbon dioxide using the same method...
Embodiment 3
[0098] Example 3: 3-bromo-1-(3-chloropyridin-2-yl)-N-(4-cyano-2-(hydroxycarbamoyl)-6-methylphenyl)-1H-pyrazole -Preparation of 5-formamide (compound 1-9):
[0099]
[0100] Compound I-9 was prepared from intermediate V-1) by the same steps as in Preparation Example 1, wherein the reagent compound VI) was prepared with NH 2 OH·HCl instead of CH 3 ONH 2 • HCl. To 2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4-oxo-4H-benzo[d][1 , 3] Oxazine-6-carbonitrile (V-1) (50 mg, 0.11 mmol) dissolved in CH 3 A solution in CN (5 mL) was added with NH 2 OH·HCl (159.8 mg, 2.3 mmol) and Et 3 N (319.3uL, 2.3mmol), stirred at room temperature for 1 hour. The reaction mixture was filtered, and the filtrate was concentrated in vacuo and subjected to flash chromatography (dichloromethane:methanol=10:1) to give a colorless solid (15 mg, yield: 28%); 1HNMR (DMSO-d6, 300MHz ), δ: 2.14(s, 3H), 7.36(s, 1H), 7.61-7.65(dd, 1H, J=7.8Hz, J=4.8Hz), 7.75(s, 1H), 8.04(s, 1H) , 8.18-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com