Glycosylation near infrared dye as well as preparation method and application thereof
A near-infrared dye, glucose-based technology, applied in chemical instruments and methods, organic dyes, azo dyes, etc., can solve the problems of inconvenient derivatization and poor drug-like properties, and achieve the effect of being beneficial to biological detection and good drug-like properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
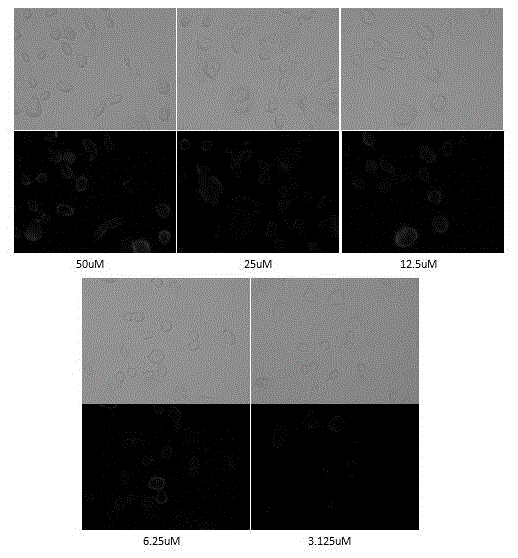
Examples
Embodiment 1
[0034] 1.1 Synthesis of compound III
[0035]
[0036] 2.0g (16.1mmol) of compound II was dissolved in 20mL of DMSO, 1.25g of sodium azide (19.2mmol) was added thereto, and heated to about 50°C for 20h. Extract with ethyl acetate, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, and distill off the solvent under reduced pressure. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:4) to obtain 1.54 g of light yellow liquid of Compound III, with a yield of 73.3%.
[0037] 1 HNMR (400MHz, CDCl 3 )δ3.69(t, J=4Hz, 2H), 3.63(t, J=4Hz, 2H), 3.55(t, J=4Hz, 2H), 3.36(t, J=6Hz, 2H), 2.67(s ,1H).
Embodiment 2
[0039] 1.2 Synthesis of compound IV
[0040]
[0041] 1.0g (8.0mmol) of compound III and 0.97mL (12.05mmol) of pyridine were dissolved in 20mL of DCM, and 1.8g (9.6mmol) of TsCl was added under ice-cooling, warmed to room temperature, and reacted for 20h. DCM extraction, dilute hydrochloric acid washing, water washing, saturated NaCl washing, anhydrous NaSO 4 After drying, the solvent was distilled off under reduced pressure. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:4) to obtain 1.1 g of compound IV as a light yellow liquid, with a yield of 48.0%.
[0042] 1HNMR (400MHz, CDCl 3 )δ7.80(d, J=8.0Hz, 2H), 7.35(d, J=8.0Hz, 2H), 4.17(t, J=5.4, 4.1Hz, 2H), 3.70(t, J=5.3, 4.1 Hz, 2H), 3.60(t, J=5.0Hz, 2H), 3.32(t, J=5.0Hz, 2H), 2.45(s, 3H).
Embodiment 3
[0044] 1.3 Preparation of compound VI
[0045]
[0046]Dissolve 5.0g (36.2mmol) of compound V in 100mL of anhydrous acetone, under nitrogen protection, add 6.5g (47.1mmol) of potassium carbonate solid in batches, after the addition is complete, add 3.3mL (38.7mmol) of MOMCl in batches, and react at room temperature for 20h . After the reaction, add water, extract with ethyl acetate, wash with saturated sodium chloride solution, dry with anhydrous sodium sulfate, and distill under reduced pressure to remove the solvent. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:4) to obtain 4.2 g of compound VI as a white solid, with a yield of 64.2%.
[0047] 1 HNMR (400MHz, CDCl 3 )δ6.12(s,2H),6.08–5.98(m,1H),5.96(s,1H),5.39(d,J=17.3Hz,1H),5.28(d,J=10.5Hz,1H), 4.65(s,2H),4.52(d,J=4.6Hz,2H),4.08(d,J=11.2Hz,2H),3.92(s,2H),3.85-3.75(m,8H),2.37-2.29 (m, 4H), 1.67-1.57 (m, 4H), 1.28 (m, 24H), 0.88 (t, J=5.9Hz, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
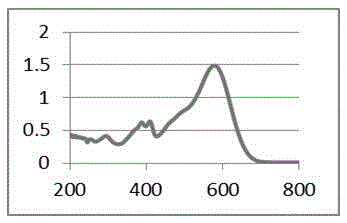
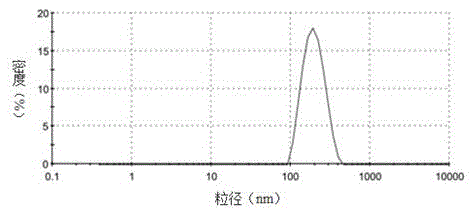
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com