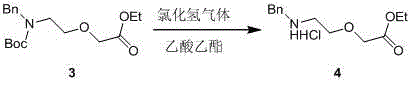Synthesis method of 4-tert-butyl-5-ethyl-6-oxo-1, 4-oxyaza-4, 5-dicarboxylic acid
A synthetic method, tert-butyl technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of lack of reports, etc., and achieve the effects of easy operation, simplified operation process, and reasonable reaction process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
[0015] 200 g compound 2 Dissolve in 1.5 liters of anhydrous tetrahydrofuran solution, add 48 grams of sodium hydride in batches under ice bath, stir for 30 minutes, add 172 grams of ethyl bromoacetate reagent dropwise, and stir at room temperature for 30 minutes. TLC (petroleum ether: ethyl acetate volume ratio = 3: 1, R f =0.5) shows that the reaction is over, the reaction solution is slowly added to a saturated ammonium chloride solution to quench the reaction, extracted with ethyl acetate, the organic layer is dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain a yellow oil, and the crude Product, yield: 70%.
Embodiment 2
[0017]
[0018] 200 g compound 2 Dissolve in 1.5 liters of anhydrous dimethylformamide solution, add 48 grams of sodium hydride in batches under ice bath, stir for 30 minutes, add 172 grams of ethyl bromoacetate reagent dropwise, and stir at room temperature for 30 minutes. TLC (petroleum ether: ethyl acetate volume ratio = 3: 1, R f =0.5) shows that the reaction is over, the reaction solution is slowly added to a saturated ammonium chloride solution to quench the reaction, extracted with ethyl acetate, the organic layer is dried by adding anhydrous sodium sulfate, filtered, and rotary evaporated under reduced pressure to obtain a yellow oil, and the crude Product, yield: 95%.
[0019] Synthesis of ethyl 2-(2-(benzylamine)ethoxy)acetate hydrochloride
[0020]
[0021] 400 g compound 3 Dissolve in 1 liter of ethyl acetate solution, slowly add 1 liter of hydrogen chloride gas / ethyl acetate (4 mol / liter) under ice bath, and stir overnight at room temperature. TLC (pet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com