A kind of preparation method of cbz valganciclovir
A preparation process and technology of lovir monoester, applied in the field of preparation of CBZ-valganciclovir, can solve the problem that the content of monoester cannot be guaranteed, the content of ganciclovir remains, and the separation of monoester and diester is difficult to achieve and other problems, to achieve the effect of reducing synthesis cost, improving conversion rate and yield, and reducing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be further described below in conjunction with specific embodiments.
[0026] Implementation column 1
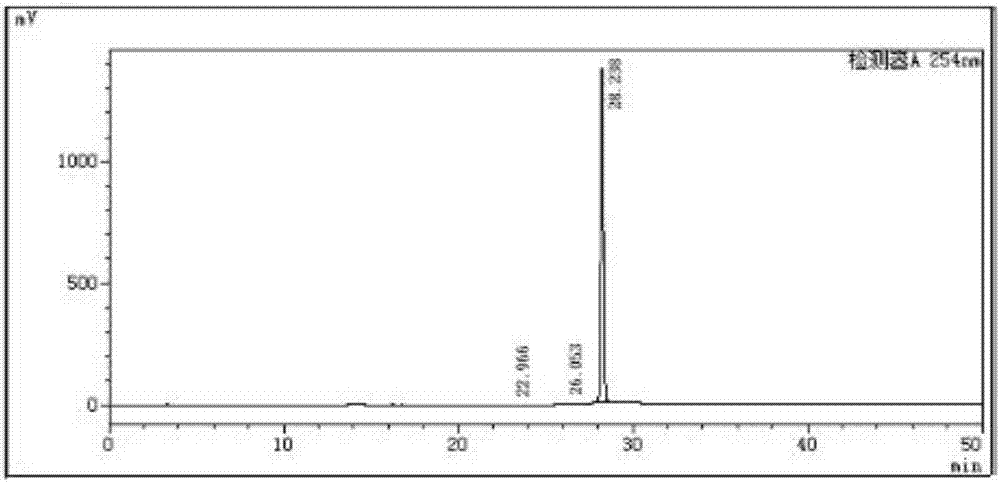
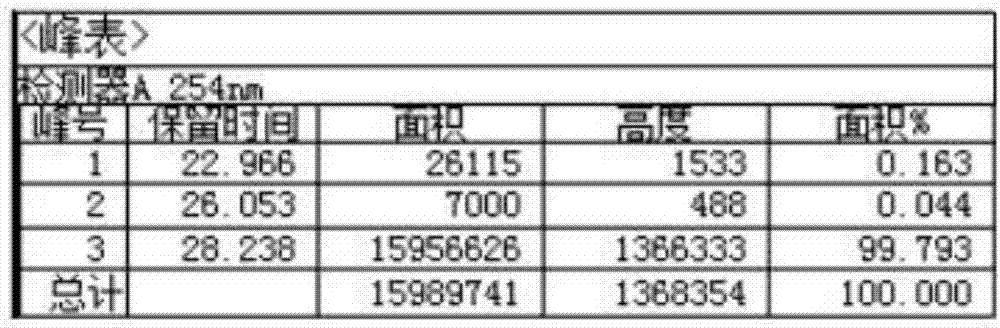
[0027] Add 50Kg of ganciclovir wet product, 200kgDMF, 200kg of trimethyl orthoacetate, and 2.5kg of p-toluenesulfonic acid into the reactor, stir and react at room temperature for 24 hours, then add 30kg of water, hydrolyze for 12 hours, distilled water and low boiling, Add 15kg of trimethyl orthoacetate, keep the temperature at 25°C and react for 3 hours. After the reaction, add 20kg of water, hydrolyze at 25°C for 2 hours, adjust the pH to neutral, distill low boiling water and water, and proceed to the next step directly with the residue To react, add 70Kg DCC and 3.7Kg DMAP to the raffinate, then add 60.0Kg CBZ-L-valine, stir at room temperature for 5-12h, add 200Kg concentration of 4N HCl aqueous solution, stir, filter with 20 mL of water washed the filter cake. The resulting filtrate was slowly adjusted to a pH of about 7-8 with a 4N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com