1, 3, 4-thiadiazole liquid crystal compound based on stilbene and preparation method of 1, 3, 4-thiadiazole liquid crystal compound
A technology of liquid crystal compounds and thiadiazoles, applied in 1 field, can solve problems such as insufficient thermal stability, and achieve the effects of excellent thermal stability, high yield, and wide temperature range of liquid crystal phases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Taking 10-HYD-F as an example to introduce the preparation of intermediate n-HYD-R
[0031] Add 308 mg (2.2 mmol) of 4-fluorobenzoic acid into 10 mL of thionyl chloride and heat to reflux for 8 h. After cooling, remove unreacted thionyl chloride to obtain 4-fluorobenzoyl chloride. 789 mg (2 mmol) of (E)-4-(2-p-decyloxyphenyl) vinylbenzoic hydrazide was dissolved in 50 mL of pyridine, and the resulting pyridine solution was added to p-fluorobenzoyl chloride. Heat to maintain the reaction system at 90°C. After 2 hours, the reaction was complete, and the solution was cooled to precipitate the product. Suction filtration gave 699 mg of white solid. Yield 71%.
[0032] The structural characterization data for the product 10-HYD-F are: 1 HNMR (400MHz, DMSO-d 6 ): δppm10.55(s, 1H, O=C-N-H), 10.50(s, 1H, O=C-N-H), 8.01(dd, J=8.8, 5.5Hz, 2H, Ar-H), 7.93(d, J= 8.3Hz, 2H, Ar-H), 7.70(d, J=8.3Hz, 2H, Ar-H), 7.57(d, J=8.7Hz, 2H, Ar-H), 7.44-7.30(m, 3H, Ar-HandC=C-H), 7.17(d, ...
Embodiment 2
[0034] Taking 10-THD-F as an example to introduce the preparation of stilbene-based 1,3,4-thiadiazole liquid crystal compound n-THD-R
[0035] Mix 516mg (1mmol) of n-HYD-F and 444mg (1.1mmol) of Lowe's reagent [bis(4-methoxyphenyl)-1,3-dithio-2,4-diphosphonane-2,4- Disulfide] was added to 50mL toluene solution, heated to reflux for 12h, and cooled to room temperature. Toluene was removed under reduced pressure, and the remaining solid was recrystallized from ethanol to obtain a crude product. The crude product was further purified through a silica gel column using chloroform as the eluent to obtain 344 mg of a light yellow solid. The yield was 67%.
[0036] The structural characterization data for the product 10-THD-F are: 1 HNMR (400MHz, CDCl 3 ): δppm8.07-7.95 (m, 4H, Ar-H), 7.59 (d, J=7.6Hz, 2H, Ar-H), 7.47 (d, J=7.6Hz, 2H, Ar-H), 7.24 -7.13(m, 3H, Ar-HandC=C-H), 7.00(d, J=16.8Hz, 1H, C=C-H), 6.91(d, J=8.0Hz, 2H, Ar-H), 3.99(t, J=6.0Hz, 2H, O-CH 2 -CH 2 ), 1.80 (m, 2...
Embodiment 3
[0038] The liquid crystal properties of the compounds of the present invention were studied by polarizing microscope (POM) and differential scanning calorimetry (DSC).
[0039] figure 1 It is the smectic A phase texture diagram of compound 10-THD-F at 325 °C during the cooling process.
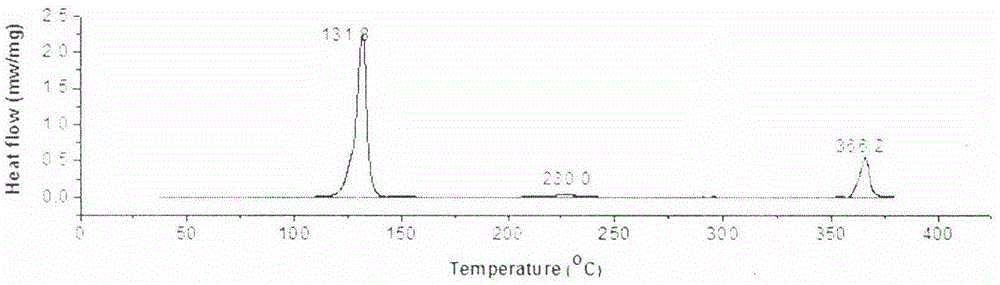
[0040] figure 2 It is the differential scanning calorimetry (DSC) diagram of compound 10-THD-F.
[0041] Table 1 shows the phase transition and the corresponding enthalpy change of compound 10-THD-R, where Cr means crystal, N means nematic phase, SmA and SmC mean smectic phase A, smectic phase C, and Iso means isotropy liquid.
[0042] Table 1 Phase transition and enthalpy change of compound 10-THD-R
[0043]
[0044] It can be seen from Table 1 that the target compounds all have a wide liquid crystal temperature range, among which 10-THD-F and 10-THD-CN have liquid crystal temperature ranges of 234.4°C and 234.0°C during the heating process, which is currently the widest liquid crysta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com