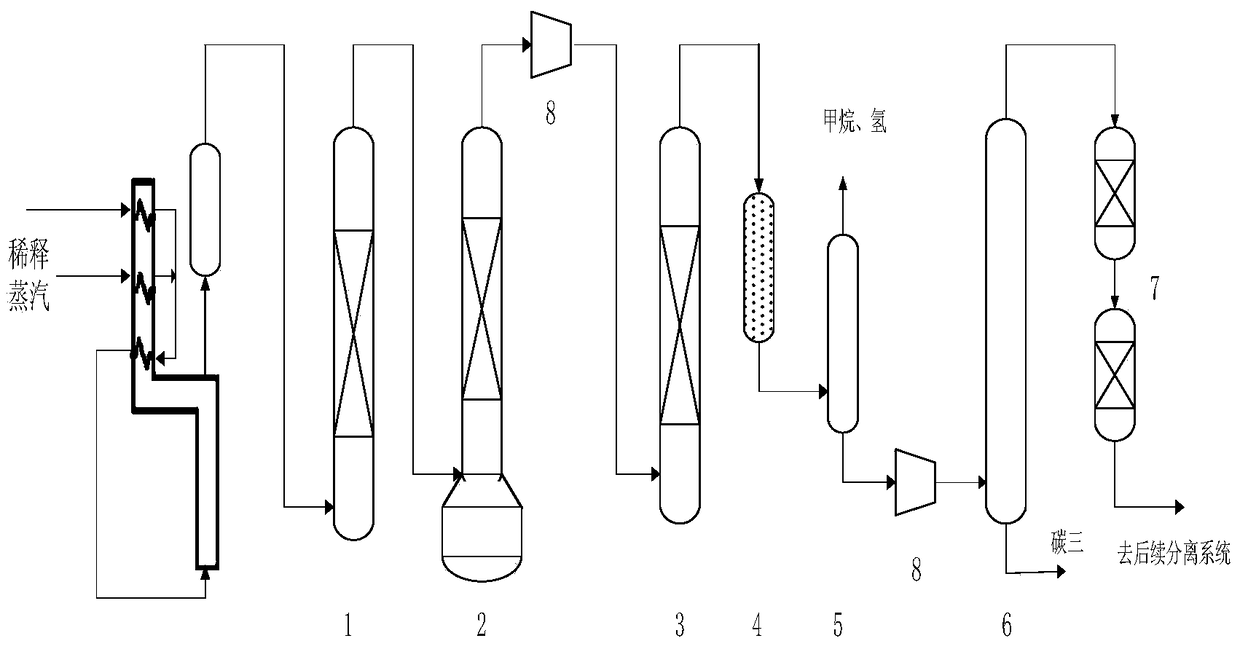
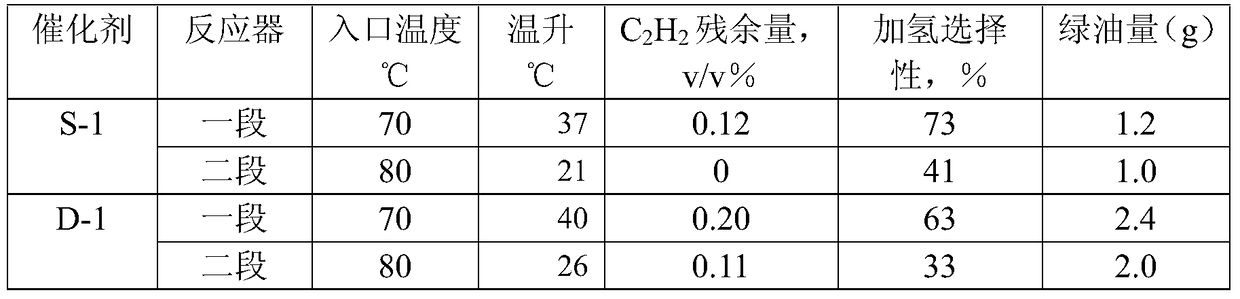
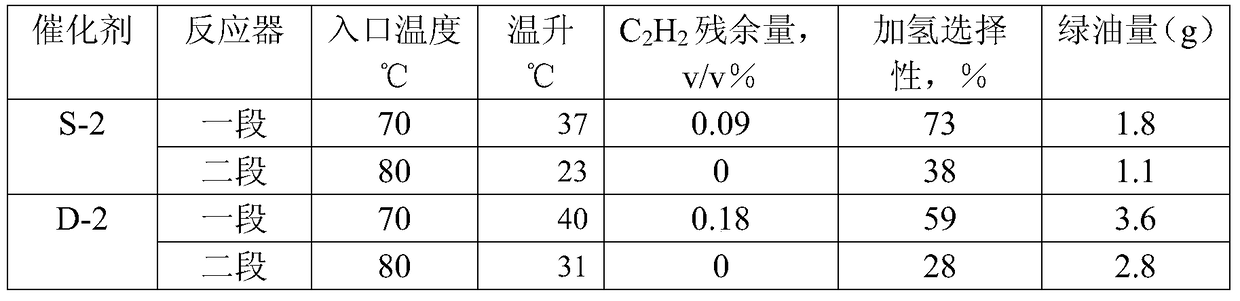
Carbon distillate post-hydrogenation method
A technology of C2 fractionation and fractionation, which is applied in the field of C2 selective hydrogenation in the sequential separation process, can solve the problems of insufficient loading of active components, increase of catalyst cost, weak complexation, etc., and achieve excellent hydrogenation The effect of hydrogen activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Weigh Φ3.5, the specific surface area is 22.0m 2 / g, pore volume 0.32mL / g, bulk density 0.80g / ml spherical α-Al 2 o 3 Carrier 500g.
[0049] Dissolve 65.39g of 4,4-dihydroxy-2,2-bipyridine in 700mL ethanol solution, impregnate the above-mentioned carrier in the above-mentioned solution, and let it stand for 2 hours to make 4,4-dihydroxy-2,2-bipyridine completely After being loaded on the alumina support, it was dried at 60°C for 10 h to obtain the hydroxyl-bipyridine / Al 2 o 3 Prebody.
[0050] Weigh 0.512g Pd(NO 3 ) 2 , 3.47g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 600mL deionized water containing an appropriate amount of nitric acid, and the pH value was adjusted to 2.1 to prepare a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 The precursor was added to the prepared solution, stirred for 10 minutes, left to stand for 2 hours, and the raffinate was poured out to obtain PdNi-hydroxy-bipyridine / Al 2 o 3 Precursor (number of moles of hydroxyl...
Embodiment 2
[0067]Weigh Φ3.6mm, height 3.6mm, specific surface area is 55.0m 2 / g, cylindrical θ-Al with a pore volume of 0.47ml / g and a bulk density of 0.70g / ml 2 o 3 Carrier 500g.
[0068] Dissolve 8.96g of 4,4-dihydroxy-2,2-bipyridine in 650mL ethanol solution, impregnate the above-mentioned carrier in the above-mentioned solution, and let the dihydroxy-2,2-bipyridine completely load on the alumina after standing for 8 hours After being mounted on the carrier, dry at 90°C for 8 hours to obtain hydroxy-bipyridine / Al 2 o 3 Prebody.
[0069] Weigh 0.84gPd(NO 3 ) 2 , 3.47gNi(NO 3 ) 2 ·6H 2 O was dissolved in 600mL deionized water containing an appropriate amount of nitric acid, and the pH value was adjusted to 2.4 to form a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let it stand for 8 hours, pour out the residual liquid, and dry the remaining solid at 110°C for 6 hours to obtain PdNi-hydroxy-...
Embodiment 3
[0088] Weigh Φ3.2mm, the specific surface area is 35.0m 2 / g, the pore volume is 0.26ml / g, the heap ratio is 0.74g / ml toothed spherical carrier 500g, wherein Al 2 o 3 460g, titanium oxide 40g, Al 2 o 3 It is a mixed crystal form of θ and α.
[0089] Dissolve 137.47g 6,6'-dihydroxy-3,3'-bipyridine in 700mL ethanol solution, impregnate the above carrier in the above solution, and let 6,6'-dihydroxy-3,3' -Bipyridine was completely loaded on the alumina support, and dried at 120°C for 4h to obtain hydroxy-bipyridine / Al 2 o 3 Prebody.
[0090] Weigh 0.683gPd(NO 3 ) 2 , 1.74g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 650mL deionized water containing an appropriate amount of nitric acid, and the pH value was adjusted to 3.0 to form a mixed solution. The above-mentioned hydroxyl-bipyridine / Al 2 o 3 Add the precursor to the prepared solution, stir for 60 minutes, let stand for 12 hours, pour out the residue, and dry at 100°C for 8 hours to obtain PdNi-hydroxy-bipyridine / Al 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com