Cobicistat pamoic acid salt and preparation method therefor, drug composition and application
A technology of pamolate and cobis, which is applied in the field of medicinal chemical crystallization, can solve the problems of difficulty in industrialization of production and strict control of carrier properties, so as to improve the uniformity of the preparation, reduce the risk of curative effect decline and safety risk, facilitate storage and transport effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0060] Preparation Example 1 Preparation of known cobicistat
[0061] The known cobicistat can be prepared according to the method described in Example 14 of patent document WO2010 / 115000A2. Specifically:
[0062] To a solution of L-thiazomorpholine ethyl ester oxalate (36.5 kg) in water (66.0 kg) was added dichloromethane (264 kg) followed by slow addition of 15 wt% KHCO 3 Solution (184.8 kg). The resulting mixture was stirred for about 1 hour. The layers were separated and the organic layer was washed with water (132 kg). The organic layer was concentrated to dryness under vacuum. Water (26.5 kg) was added to adjust the temperature of the contents to about 10°C, followed by the slow addition of 45% KOH solution (9.8 kg) while maintaining the contents at less than or equal to 20°C. At less than or equal to 20°C, the mixture was stirred until the reaction was judged complete by HPLC. The reaction mixture was concentrated to dryness under vacuum and co-evaporated with d...
preparation example 2
[0064] Preparation example 2 Preparation of known cobicistat amorphous
[0065] The known amorphous cobicistat can be prepared according to the method described in Example 1 of patent document WO2012 / 151165A1. Specifically:
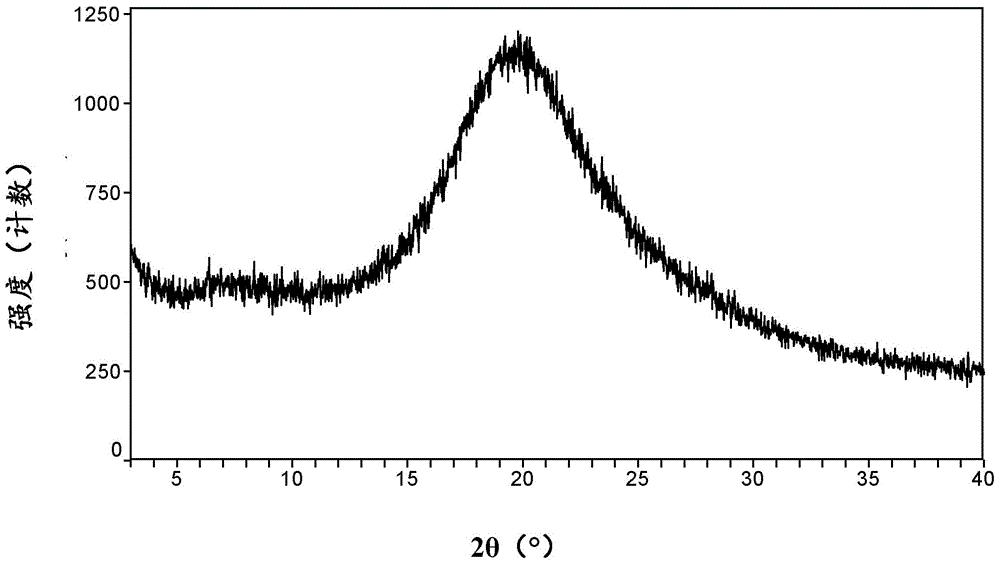
[0066] Dissolve 3.4 g of cobicistat in 12.2 g of toluene, add 122 g of toluene under stirring, stir at 5° C. for 1 hour, filter under reduced pressure, and dry in vacuum at room temperature for 24 hours to obtain the amorphous cobicistat.
[0067] XRPD patterns such as figure 1 shown.
preparation example 3
[0068] Preparation example 3 Preparation of known cobicistat 1-hydroxy-2-naphthoate
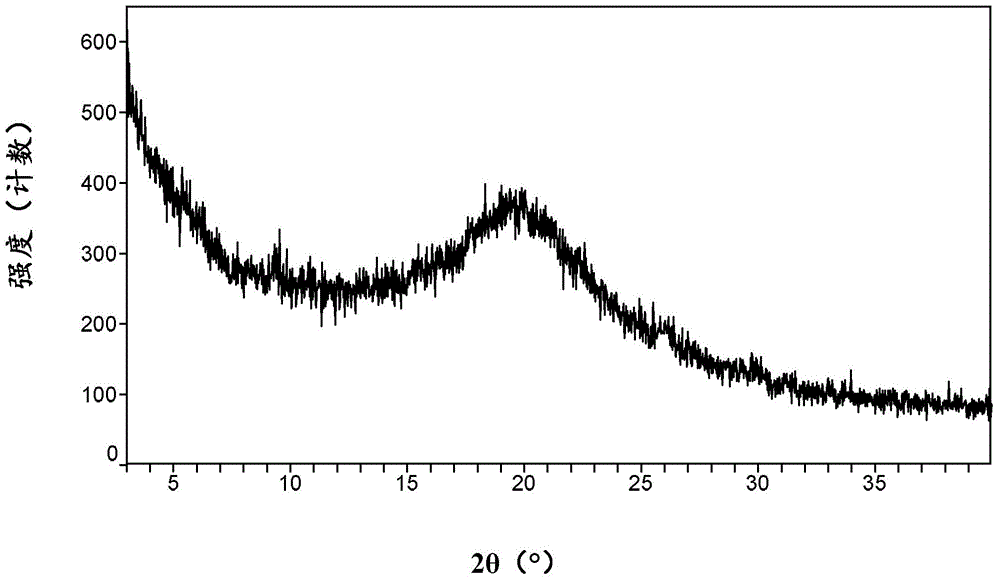
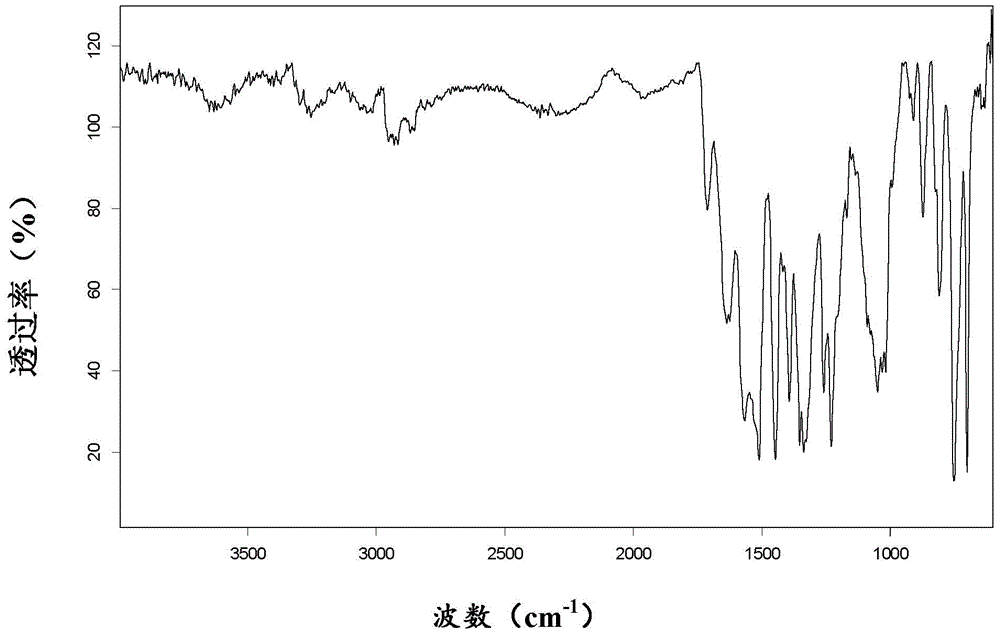
[0069] After mixing 2.0g of cobicistat and 485mg of 1-hydroxy-2-naphthoic acid, add 7mL of dichloromethane, ultrasonically dissolve, add 50mL of methyl tert-butyl ether under stirring conditions, stir at room temperature for 2 hours, and filter under reduced pressure to obtain Cobicistat 1-hydroxy-2-naphthoate.
[0070] 1 H-NMR (500MHz, d 6 -DMSO):1.31(d,6H),1.40-1.55(m,4H),1.62(m,1H),1.75(m,1H),2.10-2.35(m,6H),2.55-2.70(m,4H ),2.88(s,3H),3.22(m,1H),3.45-3.57(m,4H),3.59-3.68(m,1H),3.85-3.97(m,1H),4.08-4.10(m,1H ),4.46(s,2H),5.26(s,2H),6.60(d,1H),7.06-7.22(m,12H),7.25-7.40(m,2H),7.56(t,1H),7.58( d,1H), 7.70(d,1H), 7.96(s,1H), 8.00-8.14(m,2H), 9.07(s,1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com