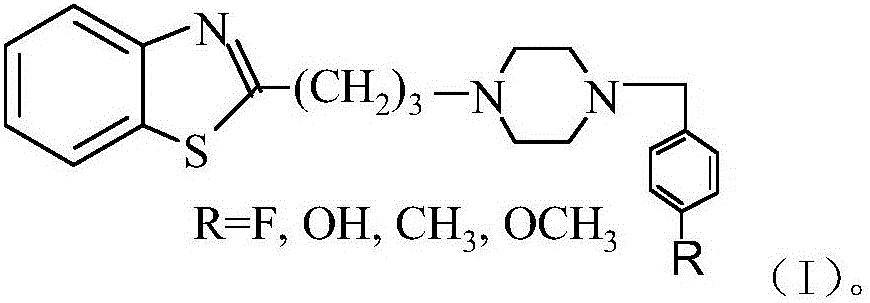Dopamine D4 receptor ligand and preparation method thereof
A dopamine and receptor technology, applied in the field of dopamine D4 receptor ligands, can solve problems such as unsatisfactory selectivity, affinity or pharmacological properties, and achieve high affinity, simple process and product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
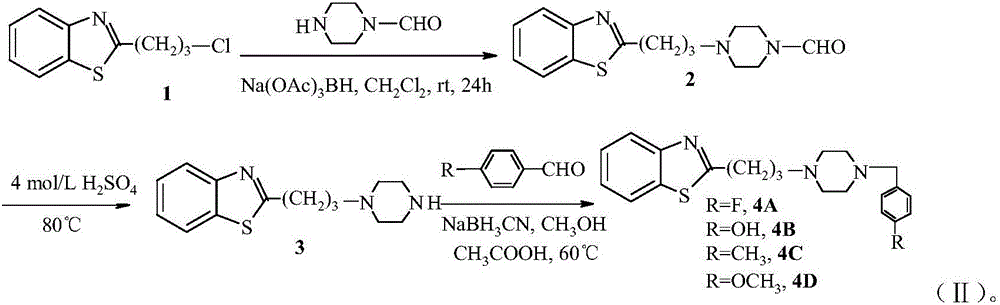
[0016] Example 1: Synthesis of 2-(3-(4-(4-fluorobenzyl)piperazin-1-yl)propyl)benzothiazole
[0017] (1) 2-(3-(4-piperazine formaldehyde-1-yl)propyl)benzothiazole
[0018] Add 0.50g (2.36mmol) of 2-(3-chloropropyl)benzothiazole and 0.50g (3.80mmol) of N-formylpiperazine to 10mL of anhydrous dichloromethane, add 1.00ml of sodium triacetoxyborohydride g (4.72mmol), stirred and reacted at room temperature for 24h, then added 20mL of ice water, extracted with dichloromethane (3×10mL), and the organic layer was dried over anhydrous sodium sulfate and purified by column chromatography to obtain 2-(3-( 4-piperazinecarbaldehyde-1-yl)propyl)benzothiazole 0.42 g, yield: 61.7%. 1 HNMR (CDCl 3 ,500MHz) δ: 7.91(d,J=8.0Hz,1H),7.83(s,1H),7.50(d,J=8.0Hz,1H),7.40(t,J=8.0Hz,1H),7.32( t,J=8.0Hz,1H),3.60(t,J=9.4Hz,2H),3.36(m,J=6.8Hz,2H),3.16-3.20(m,4H),2.70-2.75(m4H), 2.26-2.32(m,2H); ESIMSm / z(%)290.4(M+1 + , 100); elemental analysis, measured value (calculated value), %: C62.11 (62.26), H6.6...
Embodiment 2
[0023] Example 2: Synthesis of 2-(3-(4-(4-hydroxybenzyl)piperazin-1-yl)propyl)benzothiazole
[0024] Dissolve 2-(3-(4-piperazin-1-yl)propyl)benzothiazole (0.10 g, 0.38 mmol) in methanol (10 mL), add p-hydroxybenzaldehyde 70 μL (0.65 mmol), acetic acid ( 80μL), sodium cyanoborohydride (0.050g, 0.80mmol), stirred at room temperature for 5 hours, extracted the mixture with dichloromethane and water, dried the organic layer over anhydrous sodium sulfate, concentrated under reduced pressure, and passed the crude product through a Flash column After chromatographic separation, 0.12 g of 2-(3-(4-(4-hydroxybenzyl)piperazin-1-yl)propyl)benzothiazole was obtained with a yield of 85.4%. 1 HNMR (CDCl 3 ,500MHz) δ: 9.32(s,1H),7.92(d,J=7.5Hz,1H),7.50(d,J=9.3Hz,1H),7.43(t,J=6.2Hz,1H),7.40( q, J=5.0Hz, 2H), 7.32(t, J=6.5Hz, 1H), 7.20(t, J=8.0Hz, 2H), 3.65(t, J=9.4Hz, 2H), 3.35(s, 2H),3.26(s,2H),3.12-3.19(m,4H),2.72-2.77(m4H),2.25-2.30(m,2H);ESIMSm / z(%)368.5(M+1 + , 100); elemental analysi...
Embodiment 3
[0025] Example 3: Synthesis of 2-(3-(4-(4-methylbenzyl)piperazin-1-yl)propyl)benzothiazole
[0026] Dissolve 2-(3-(4-piperazin-1-yl)propyl)benzothiazole (0.10g, 0.38mmol) in methanol (10mL), add p-tolualdehyde 70μL (0.59mmol), acetic acid (80μL), sodium cyanoborohydride (0.050g, 0.80mmol), stirred at room temperature for 5 hours, extracted the mixture with dichloromethane and water, dried the organic layer over anhydrous sodium sulfate, concentrated under reduced pressure, and the crude product was flash After separation by column chromatography, 0.09 g of 2-(3-(4-(4-methylbenzyl)piperazin-1-yl)propyl)benzothiazole was obtained with a yield of 64.3%. 1 HNMR (CDCl 3 ,500MHz) δ: 7.93(d,J=8.0Hz,1H),7.49(d,J=8.0Hz,1H),7.45(t,J=8.5Hz,1H),7.42(q,J=5.5Hz, 2H), 7.30(t, J=8.2Hz, 1H), 7.18(t, J=8.6Hz, 2H), 3.66(t, J=8.0Hz, 2H), 3.32(s, 2H), 3.25(m, J=6.0Hz, 2H), 3.15-3.20(m, 4H), 2.72-2.75(m4H), 2.32(s, 3H), 2.20-2.24(m, 2H); ESIMSm / z(%)366.5(M+ 1 + , 100); Elemental analysis, meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com