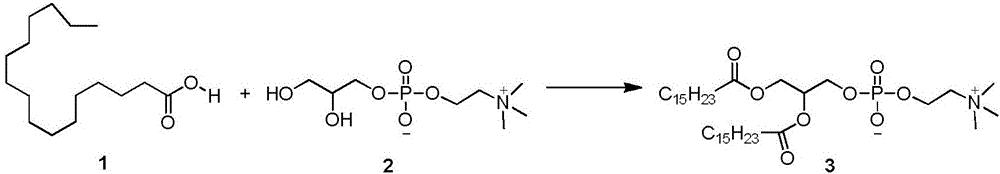
Preparation method of synthetic phospholipid DPPC (dipalmitoyl phosphatidylcholine)
A technology for synthesizing phospholipids and palmitoylphosphatidylcholine, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome post-treatment process, high toxicity of reaction solvents, and serious environmental pollution, and achieve cheap raw materials, easy industrial production, and high economic benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
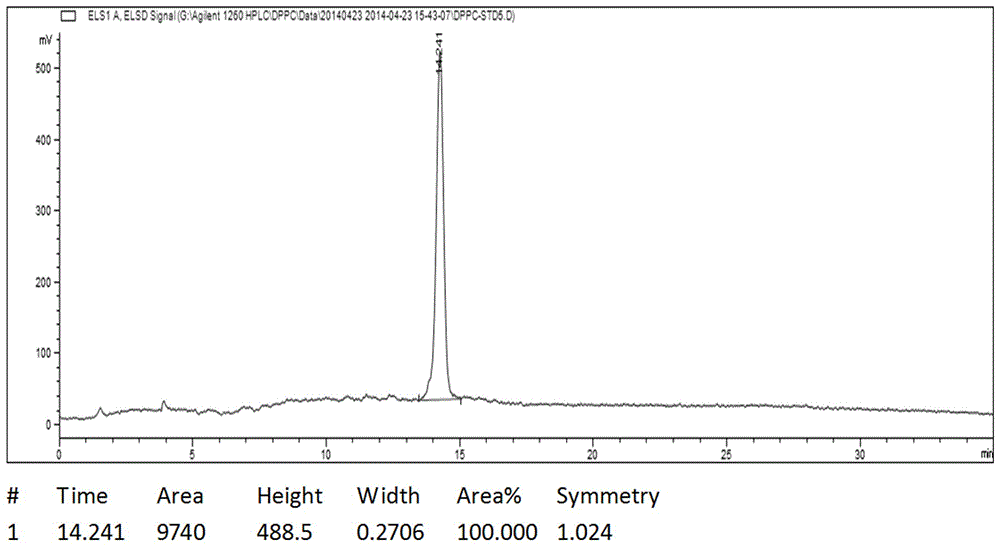
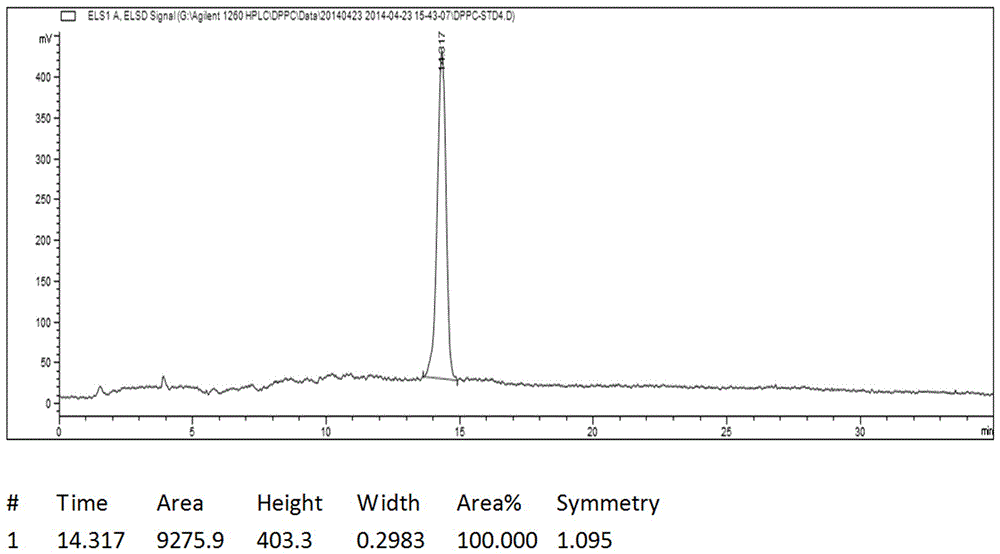
Image
Examples
Embodiment 1
[0029] Add 50g glycerol phosphonocholine, 200g palmitic acid, 95g DMAP, and 1000mL chloroform to a clean and dry 2000mL three-necked flask. Stir evenly with magnetic force, and protect with argon replacement. Dissolve 165g of DCC in 1000mL of chloroform, and quickly drop the DCC chloroform solution into the reaction system. Avoid light and react at 20°C for 5h. During the reaction process, a large amount of white insoluble solids appeared, and the reaction was stopped if no further progress was detected by TLC. The reaction solution was filtered to remove the white insoluble solid DCU to obtain a bright yellow transparent liquid, which was evaporated to dryness to obtain a yellow viscous liquid, which was beaten with 3000 mL of acetone for 10 h, and a large amount of white solids were precipitated in the system. The catalyst 4-dimethylaminopyridine (DMAP) in the system was removed by filtration. Repeat once, filter off-white solid, and dry to obtain crude product 130g. Hea...
Embodiment 2
[0033] Add 5g of glycerylphosphonocholine, 20g of palmitic acid, 9.5g of TEA, and 50mL of chloroform into a clean and dry 200mL three-necked flask. Stir evenly with magnetic force, and protect with argon replacement. Dissolve 16.5 g of DCC in 200 mL of chloroform, and quickly drop the DCC chloroform solution into the reaction system. Protect from light and react below 20°C for 1d. During the reaction process, a large amount of white insoluble solids appeared, and the reaction was stopped if no further progress was detected by TLC. The reaction solution was filtered to remove the white insoluble solid DCU to obtain a bright yellow transparent liquid, which was evaporated to dryness to obtain a yellow viscous liquid, which was beaten with 300 mL of acetone for 10 hours, and a large amount of white solid was precipitated in the system, filtered to obtain an off-white solid, and dried to obtain Crude product 13g. Heat the crude product with 200mL of dichloromethane at 50°C with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com