A connecting peptide and its application
A technology for linking peptides and heparinase, applied in the fields of genetic engineering and fermentation engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Design of linker peptide, construction and expression of target linker peptide MBP fusion polysaccharide lyase vector
[0048] 1. Design of connecting peptide
[0049] (1) The origin of the connecting peptide:
[0050] Since the length and properties (rigidity and flexibility) of the connecting peptide can significantly affect the properties of the fusion protein, this is mainly due to the change of the spatial distance and positional relationship between the two elements in the fusion protein, resulting in the mutual force and space The difference in steric hindrance will affect the structure of the protein to different degrees, and finally affect the biological function of the two elements, especially the biological function of the main catalytic enzyme. Length has the most significant impact on the properties of fusion proteins. However, for this reason, it is difficult to predict to what extent changes in length will affect fusion proteins, so it is very ...
Embodiment 2
[0055] Expression, purification and enzyme activity analysis of embodiment 2 target protein
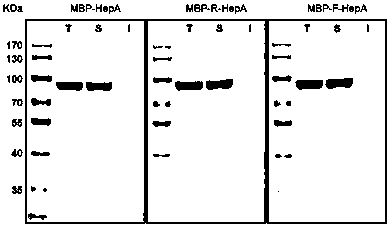
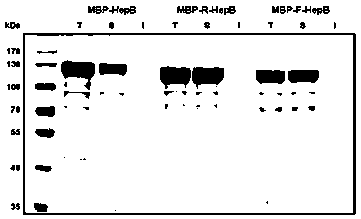
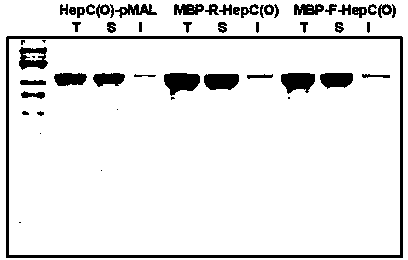
[0056] Expression of polysaccharide lyase vector containing target connecting peptide fusion: transform the constructed plasmid containing target connecting peptide fusion polysaccharide lyase vector into Escherichia coli respectively, and shake the strain transformed with recombinant plasmid in LB medium containing 100ug / mL ampicillin After culturing overnight (37°C, 180rpm, 14-16h), inoculate into LB / M9YE fermentation medium at a ratio of 1:100, shake at 37°C and 180rpm to the logarithmic growth phase, add IPTG and turn to 200rpm, 15°C Low temperature induction culture for 20-22h.
[0057] Protein extraction and purification process: Collect 100ml of the target Escherichia coli fermentation broth and resuspend with 20ml of protein extraction buffer, ultrasonically disrupt the bacteria, centrifuge the broken product at 10,000rpm for 30 minutes at 4°C, collect the supernatant, and fil...
Embodiment 4
[0093] Example 4 Thermal stability analysis of MBP fusion polysaccharide lyase before and after the replacement of the connecting peptide
[0094] Measure the enzyme activity immediately after protein purification as the enzyme activity at time 0, place the pure enzyme in a water bath at the detection temperature, select different time points to sample and measure the enzyme activity, compare with the enzyme activity at time 0, and calculate the time Relative to the percentage of enzyme activity at time 0. Continue this operation until the enzyme activity drops to a lower level, so that a series of corresponding relationship points between warming time points and remaining enzyme activities are obtained, and these points are connected to obtain the inactivation curve of the enzyme at this temperature, that is, The stability of the enzyme at this temperature can be evaluated.
[0095] 1. Analysis of enzyme thermostability after shaking flask fermentation before and after repla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com