Imidazo pyridine rhodamine hydrazide type cupric ion ratio fluorescence probe and application thereof
A ratiometric fluorescent probe and rhodamine hydrazide technology, which is applied in the fields of fluorescence/phosphorescence, luminescent materials, and material analysis through optical means, can solve the problem of fewer ratiometric fluorescent probes and achieve strong anti-interference ability of other ions , enhanced fluorescence intensity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: The synthesis scheme of the compound of formula (1) is shown in the following formula:
[0016]
[0017] Concrete synthetic steps are as follows:
[0018] In a 50 mL round bottom flask, add 0.253 g (1.0 mmol) imidazo[1,5-a] pyridinecarboxylic acid, 0.290 g (1.5 mmol) 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine (EDC), 0.180g (1.5mmol) 4-dimethylaminopyridine (DMAP), 20mL anhydrous dichloromethane, react at room temperature for 30 minutes, then add 0.470g (1.0mmol) rhodamine hydrazide, room temperature The reaction was carried out for 10 hours. After the reaction was detected by TLC, 100 mL of dichloromethane was added, washed three times with 30 mL of water, the dichloromethane layer was dried over sodium sulfate, concentrated, and column chromatography gave 0.48 g of an off-white solid with a yield of 68.2%.
[0019] H NMR spectrum determination: 1 HNMR (400MHz, CDCl 3 ): δ7.95(m,1H),7.72(dd,J=4.0Hz,8.0Hz,1H),7.51-7.46(m,3H),7.10-7.08(m,1H),6.7...
Embodiment 2
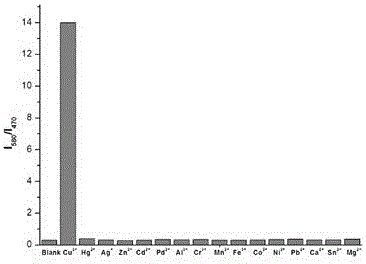
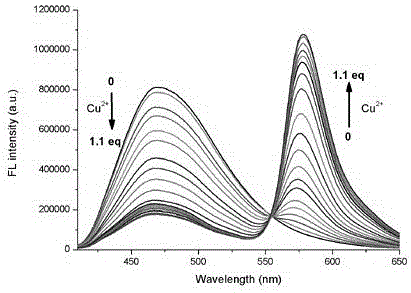
[0021] To formula (1) compound (10 -5 M) were added 2 equivalents of Cr in the ethanol solution 3+ , Mn 2+ , Fe 3+ ,Co 2+ , Ni 2+ ,Cu 2+ ,Zn 2+ ,Cd 2+ ,Hg 2+ and Ag + After measuring the change in the ratio of fluorescence emission intensity at 470nm and 580nm, it was found that: the compound of formula (1) has 2+ Has a unique fluorescence selectivity, adding 1 equivalent of Cu 2+ After that, the fluorescence intensity of compound 1 at 470nm was significantly reduced, and at the same time, the fluorescence intensity at 580nm was significantly enhanced, I 580 / I 470 =13.99, such as figure 2 shown.
Embodiment 3
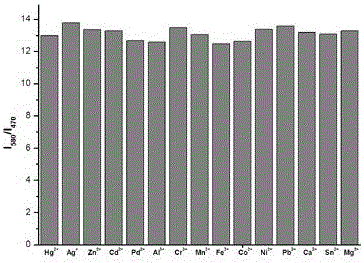
[0023] In formula (1) compound (10 -5 M) and 1 equivalent of Cu 2+ 2 equivalents of Mg were added to the ethanol solution 2+ , Ca 2+ , Al 3+ ,Sn 2+ ,Pb 2+ ,Cr 3+ , Mn 2+ , Fe 3+ ,Co 2+ , Ni 2+ ,Zn 2+ ,Cd 2+ ,Pd 2+ ,Hg 2+ and Ag + After measuring the change in the ratio of fluorescence emission intensity at 470nm and 580nm, it was found that the compound of formula (1) has strong anti-interference ability to other ions, such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com