A kind of preparation method of recombinant baculovirus vaccine for prevention and treatment of avian influenza h5n1
A technology of recombinant baculovirus and avian influenza virus, which is applied in the field of preparation of recombinant baculovirus vaccines, can solve the problems of short duration of foreign gene expression, low efficiency and gene expression level, and restrictions on the application of baculovirus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Cloning of embodiment 1.HA gene
[0038] Lymphocytes were isolated from chicken peripheral blood, and after culturing for 8 hours, the genomic RNA of chicken lymphocytes was extracted using the Trizol Reagent kit, reverse-transcribed to obtain the first strand of cDNA, and the chicken HA gene was cloned by PCR, which was connected to pMD18- On the T vector, it was named "pT-HA" after verification and correct sequencing.
[0039] 1.1 Culture of Chicken Peripheral Blood Lymphocytes
[0040]Use a pipette to add 2 mL of lymphocyte separation solution to the bottom of a 15 mL centrifuge tube, avoiding contact with the side wall. Anticoagulant needles Take 3mL of chicken wing underarm blood, add 3mL D-Hanks solution, mix with a pipette, pay attention to avoid air bubbles, slowly add to the lymphocyte separation medium, 2000r / min, after centrifugation for 20min, use Aspirate the buffy coat layer with a 1mL syringe, draw as little separation liquid as possible, and place it...
Embodiment 2
[0057] Construction of embodiment 2.pT-HA plasmid
[0058] After the bands were detected correctly, the remaining PCR products were recovered and purified, and the specific operation steps were performed according to the instructions of the GelExtraction Mini Kit (Tiangen, M2029). Add "A" to the HA fragments recovered from the gel, the reaction system is shown in Table 6, and the reaction program settings are shown in Table 7. The recovered product after treatment was connected to the pMD18-T vector overnight at 16°C. The reaction system is shown in Table 8. The correct positive plasmid was named "pT-HA".
[0059] Table 6 Reaction system of adding "A" to the end of HA of PCR amplification product
[0060]
[0061] Table 7 PCR amplification product HA plus "A" reaction program
[0062]
[0063] Table 8 Reaction system of ligation of HA fragment and pMD18-T vector
[0064]
Embodiment 3
[0065] Construction of embodiment 3.pS-ITRs-HA plasmid
[0066] The pS-ITRs-HA plasmid contains CMV promoter and regulatory elements such as WPRE, VSV-GED, ITRs and gp64sp, which were respectively obtained from the template plasmid pEGFP-C3 (purchased from Clontech Company), pRRLSIN. Addgene), pCMV-VSVG (purchased from addgene), pAAV-LacZ (purchased from Agilent) and Bacmid were cloned and identified.
[0067] 3.1 Cloning of each component
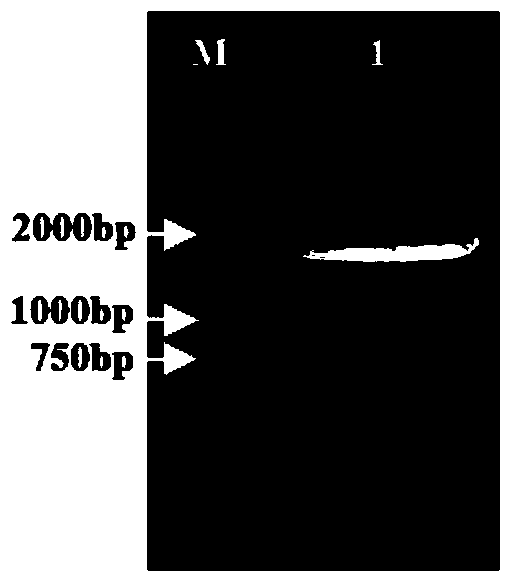
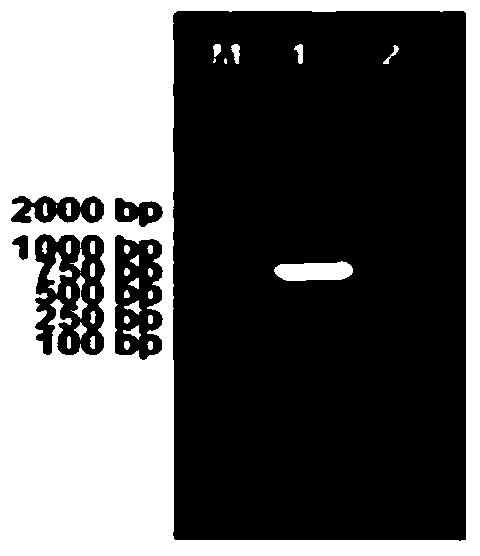
[0068] Using plasmids pEGFP-C3, pRRLSIN.cPPT.PGK-GFP.WPRE, pCMV-VSVG, pAAV-LacZ and Bacmid as templates, CMV-up, CMV-down, WPRE-up, WPRE-down, VSV-GED-up , VSV-GED-down, ITRs-L-up, ITRs-L-down, ITRs-R-up, ITRs-R-down and gp64sp-up, gp64sp-down as upstream and downstream primers, PCR amplification of CMV, WPRE, For genes of regulatory elements such as VSV-GED, ITRs, and gp64sp, the reaction system is shown in Table 4, and the reaction program settings are shown in Table 5. The results of PCR amplification were as figure 2 , image 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com