Quick release agent of large-dose medicine and preparation method of quick release agent
An immediate-release preparation and large-dose technology, applied in the field of medicine, can solve the problems that the preparation is not suitable for patients to take, the preparation is difficult to produce and use, and the amount of active pharmaceutical ingredients is large. Beneficial for swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of highly hydrophilic auxiliary material matrix: 100 g of sorbitol was dissolved in 1 L of depurified water; (2) under continuous stirring, 150 g of povidone was slowly added to dissolve, and then 204 g of Tween was added to obtain a highly hydrophilic auxiliary material base.
[0052] Preparation of pharmaceutical powder: mycophenolate mofetil is pulverized by jet to particle size D 90 If it is less than 20 microns, add a total amount of 1% micronized silica gel, mix well, and set aside.
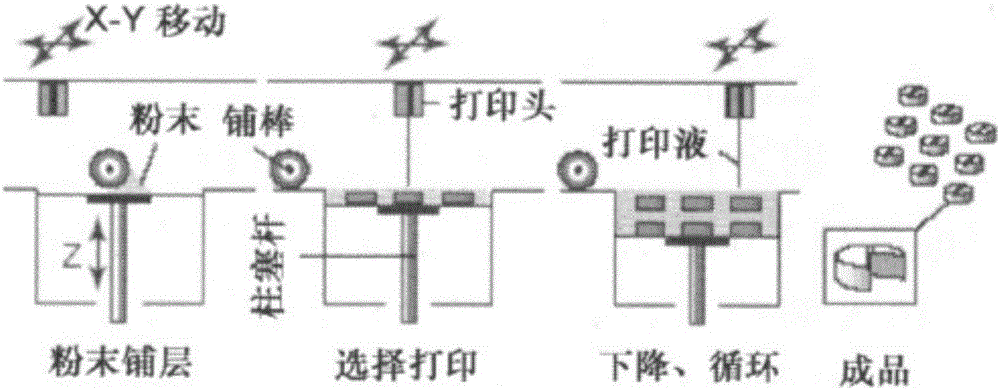
[0053] 3D printing: tablets are obtained by powder bond printing. The active ingredient content is 99%, and the tablet weight is 1010mg.
Embodiment 2
[0054] [Example 2] Preparation of sofosbuvir+velpatasvir+GS-9857 compound tablet
[0055] Preparation of highly hydrophilic adjuvant matrix: 100 g of sorbitol was dissolved in 1 L of 80% ethanol; (2) under continuous stirring, 50 g of hydroxypropyl cellulose was slowly added to dissolve, and then 2 g of Span was added to obtain a highly hydrophilic adjuvant matrix.
[0056] Pharmaceutical powder preparation: Three components are jet milled to particle size D 90 If it is less than 1nm, add an appropriate amount of highly hydrophilic auxiliary material matrix, mix evenly, and use it as a printing material for later use.
[0057] 3D printing: printing materials, tablets are obtained by direct printing. The active ingredient content is 98%, and the tablet weight is 1.2g.
Embodiment 3
[0058] [Example 3] Preparation of Capecitabine Tablets
[0059] Preparation of highly hydrophilic auxiliary material matrix: 200g mannitol alcohol is dissolved in 1L80% ethanol; (2) under continuous stirring, slowly add hypromellose 10g to dissolve, then add sodium lauryl sulfate 40g, obtain high Hydrophilic excipient matrix.
[0060] Pharmaceutical powder preparation: to particle size D 90 If it is less than 1 micron, add a total of 1% micronized silica gel and mix evenly, and set aside.
[0061] 3D printing: tablets are obtained by powder bond printing. The active ingredient content is 90%, and the tablet weight is 550mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com