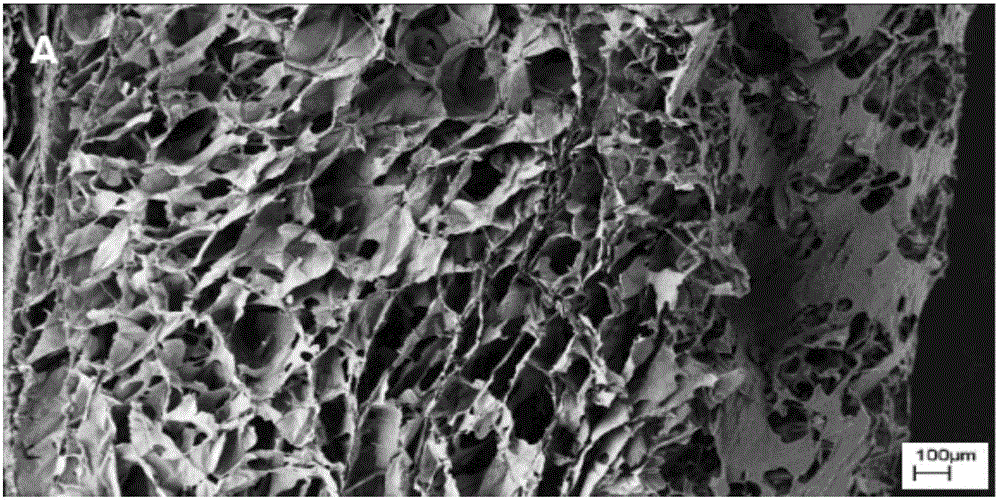
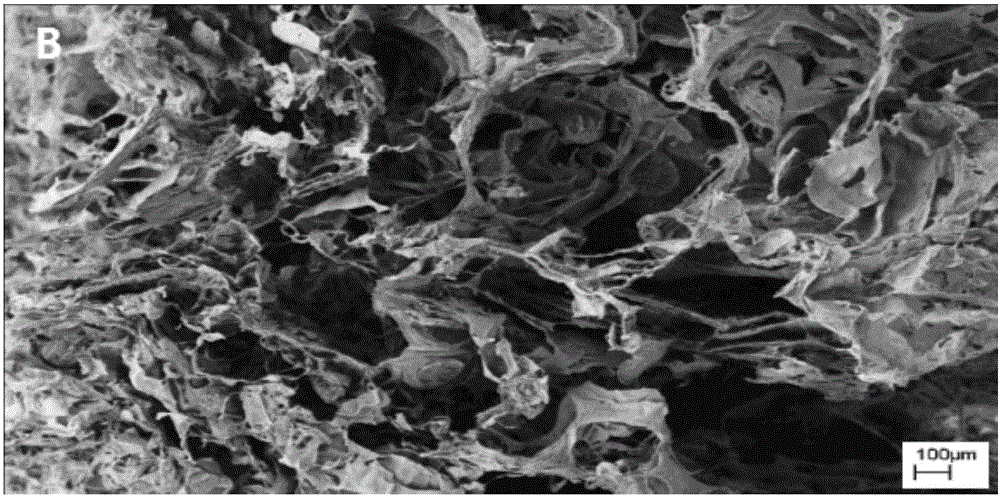
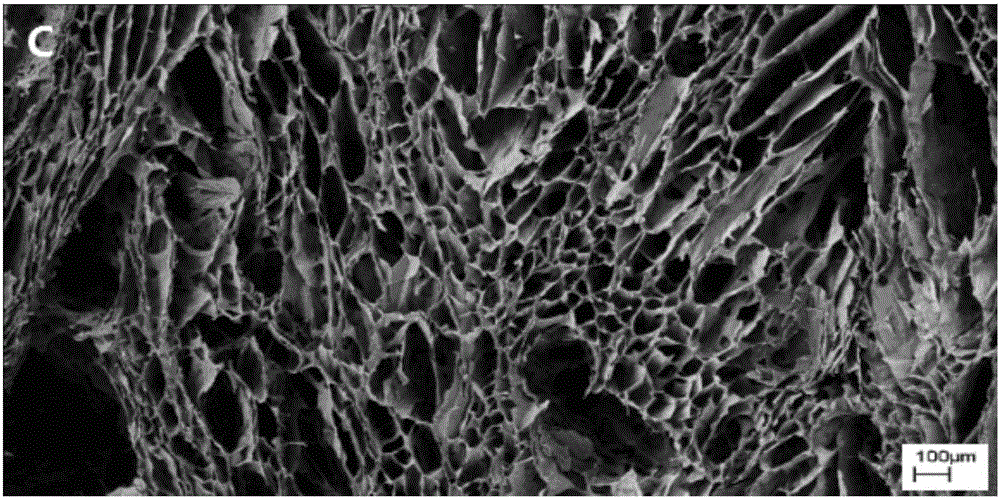
Application of dodecyl chitosan in preparing hemostasis dressing
A dodecyl shell and hemostatic dressing technology, applied in medical science, absorbent pads, bandages, etc., can solve problems such as delayed wound healing, unstable intermediate Schiff base, and fibrin glue derived from blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of dodecyl chitosan powder, comprises the steps:
[0041] (1) dissolving chitosan (CS) with a viscosity-average molecular weight of 650,000 and a degree of deacetylation of 95% in an aqueous acetic acid solution with a volume concentration of 1%, stirring until completely dissolved; the chitosan and the aqueous acetic acid solution The ratio is 2g:100mL;
[0042] (2) Add 1mol / L aqueous sodium hydroxide solution to the solution obtained in step (1) to adjust the pH to 12 to produce chitosan precipitation; filter and dissolve the filter cake in dimethylformamide; the filter cake product The ratio with dimethylformamide is 5g:150mL; add lauric aldehyde (Aladdin, Shanghai) according to the ratio of the number of chitosan repeating unit molecules to the number of lauric aldehyde molecules, and adjust the pH with acetic acid to 6.5, stirred at room temperature for 6 h; under nitrogen, added sodium triacetoxyborohydride (STAB-H, Kramar, Shanghai) equimo...
Embodiment 2
[0047] The preparation method of dodecyl chitosan powder, comprises the steps:
[0048] (1) be 300,000, the chitosan of degree of deacetylation 92% to be dissolved in the acetic acid aqueous solution that volume concentration is 1% by viscosity-average molecular weight, stir until fully dissolving; The ratio of described chitosan and described acetic acid aqueous solution is 2g: 50mL;
[0049] (2) Add 1mol / L aqueous sodium hydroxide solution to the solution obtained in step (1) to adjust the pH to 8 to produce chitosan precipitation; filter and dissolve the filter cake in dimethylformamide; the filter cake product The ratio with dimethylformamide is 5g:100ml; the ratio of the number of chitosan repeating unit molecules to the number of laurylaldehyde molecules is 5:1, add lauric aldehyde, adjust the pH to 7 with acetic acid, and stir at room temperature 4h; add sodium triacetoxyborohydride equimolar with laurylaldehyde under nitrogen, continue to react for 12h, filter, filter...
Embodiment 3
[0053] The preparation method of dodecyl chitosan powder, comprises the steps:
[0054] (1) be 1,000,000, the chitosan of degree of deacetylation 98% to be dissolved in the acetic acid aqueous solution that volume concentration is 1% by viscosity-average molecular weight, stir until fully dissolving; The ratio of described chitosan and described acetic acid aqueous solution is 2g: 100mL;
[0055] (2) Add 1mol / L aqueous sodium hydroxide solution to the solution obtained in step (1) to adjust the pH to 10 to produce chitosan precipitation; filter and dissolve the filter cake in dimethylformamide; the filter cake product The ratio with dimethylformamide is 5g:200ml; Add laurylaldehyde according to the ratio of 10:3 between the number of chitosan repeating unit molecules and the number of laurylaldehyde molecules, adjust the pH to 5 with acetic acid, and stir at room temperature 8h; Add sodium triacetoxyborohydride equimolar with laurylaldehyde under nitrogen, continue to react f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com