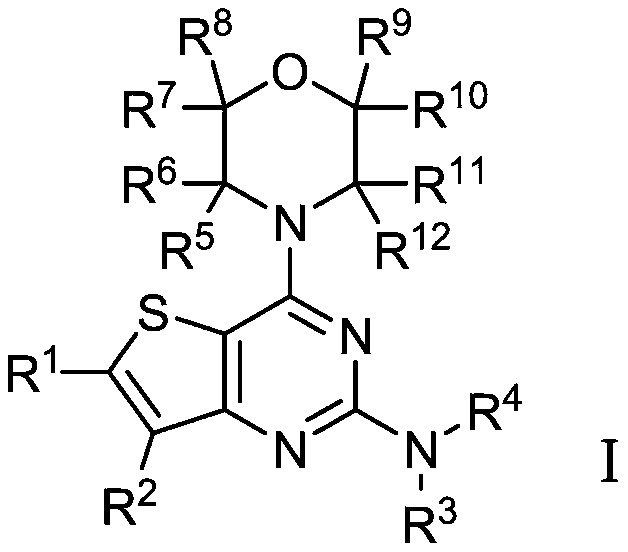
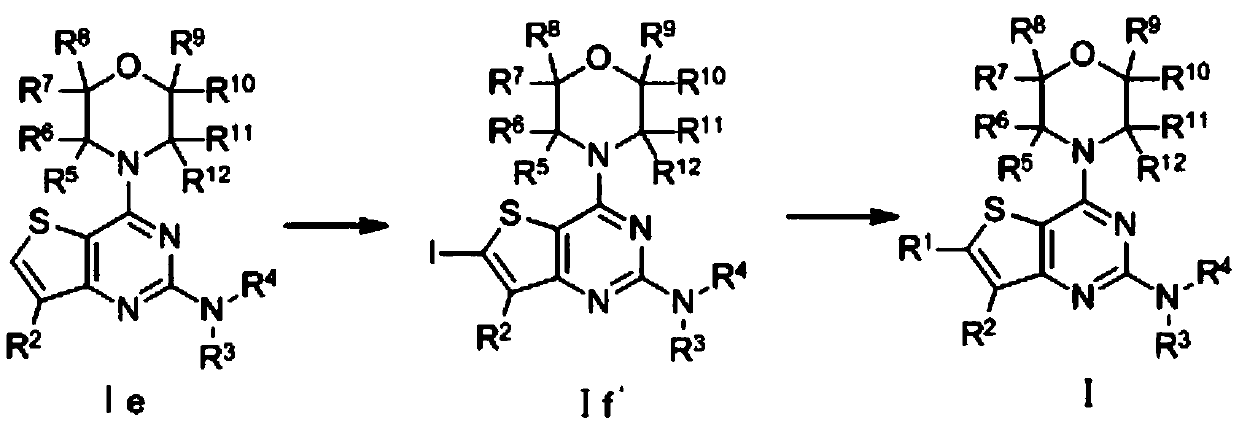
A kind of thienopyrimidine compound and its preparation method and application
A compound and pyrimidine technology, applied in the field of thienopyrimidine compounds and their preparation, can solve problems such as physical hazards, toxic side effects, etc., and achieve the effect of preventing and treating cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
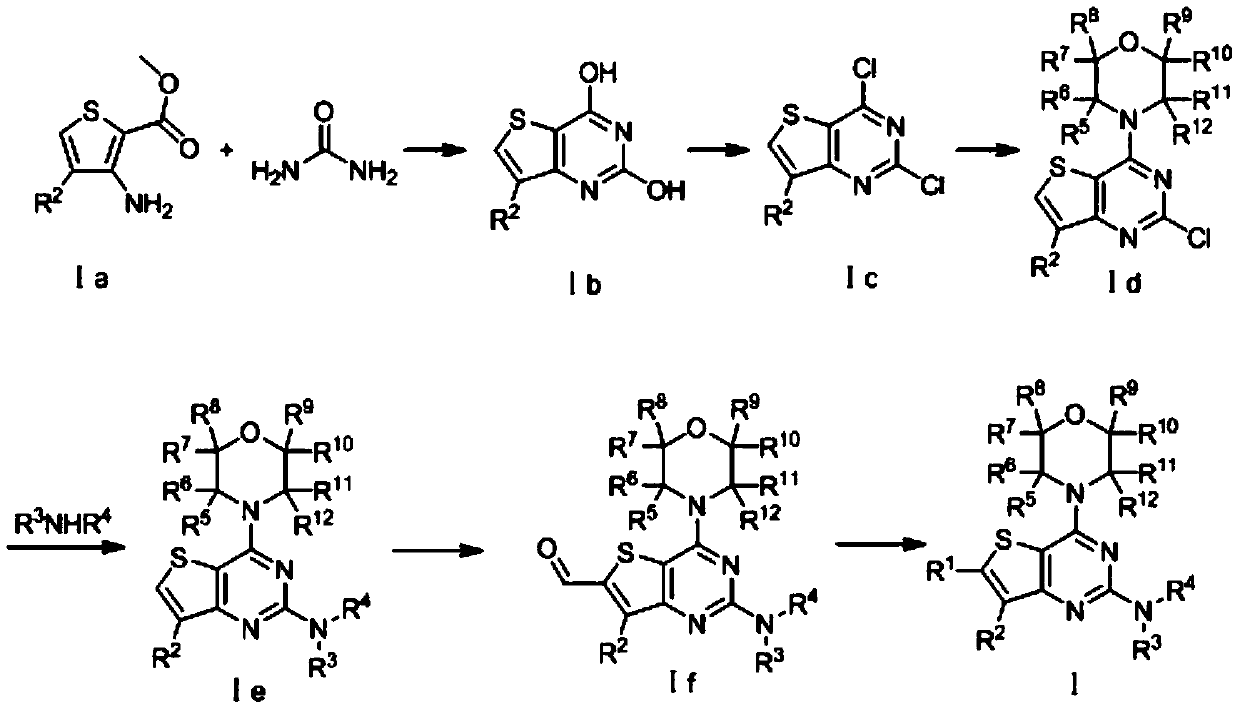
[0037] A thienopyrimidine compound: (2-((2S,6R)-2,6-dimethylmorpholinyl)-4-morpholinothieno[3,2-d]pyrimidin-6-yl) Preparation of Methanol: The synthetic route is as follows:
[0038]
[0039] 100 mmol of methyl 3-amino-2-thiophenecarboxylate and 500 mmol of urea were placed in a microwave reactor at 200°C for 30 min to obtain yellow crystalline compound Ib-1. 50 mmol of compound Ib-1 was added to 100 ml of phosphorus oxychloride mixed solution and refluxed at 110° C. for 10 hours to obtain the chlorinated product compound Ic-1. Suspend 34mmol of compound Ic-1 in 150ml of ethanol, add 100mmol of morpholine at a stirring speed of 100r / min, and react at room temperature for 2h to obtain compound Id-1. Mix 30mmol of compound Id-1, 90mmol of trifluoroacetic acid, and 45mmol of (2S,6R)-2,6-dimethylmorpholine in 100ml of n-butanol and reflux at 120°C for 3h to obtain compound Ie-1. Dissolve 20mmol of compound Ie-1 in 100ml of tetrahydrofuran, under nitrogen protection, add 24mm...
Embodiment 2
[0042] A kind of thienopyrimidine compound: the preparation of (4-morpholine-2-thiomorpholinothieno[3,2-d]pyrimidin-6-yl)methanol: the synthetic route is as follows:
[0043]
[0044] On the basis of Example 1, 10 mmol of compound Id-1, 30 mmol of trifluoroacetic acid, and 15 mmol of thiomorpholine were mixed in 30 ml of n-butanol and refluxed for 3 hours at 120 ° C to obtain compound Ie-2 . Dissolve 20mmol of compound Ie-2 in 100ml of tetrahydrofuran, under nitrogen protection, add 8mmol of Grignard reagent dropwise at -78°C, after the addition is complete, stir and react at a stirring speed of 50r / min for 2h, then add 15ml of DMF, and continue stirring for 4h Compound If-2 was obtained; at room temperature, 2 mmol of Compound If-2 was reduced with sodium borohydride in dichloromethane. After the reaction was completed, the reaction organic phase was washed three times with saturated brine, dried over anhydrous sodium sulfate, and subjected to silica gel column chromatogra...
Embodiment 3
[0047] A thienopyrimidine compound: the preparation of (2-(indol-1-yl)-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methanol: the synthetic route is as follows:
[0048]
[0049] On the basis of Example 1, 10 mmol of compound Id-1, 30 mmol of trifluoroacetic acid, and 15 mmol of indoline were mixed in 30 ml of n-butanol and refluxed at 130°C for 3.5 hours to obtain compound Ie- 3. Dissolve 20mmol of compound Ie-3 in 100ml of tetrahydrofuran, under nitrogen protection, add 8mmol of n-butyllithium dropwise at -78°C, after the addition is complete, stir and react at a stirring speed of 60r / min for 2h, then add 15ml of DMF, and continue stirring Compound If-3 was obtained after reaction for 4 hours; at room temperature, 2 mmol of compound If-3 was reduced with sodium borohydride in dichloromethane. After the reaction was completed, the reaction organic phase was washed with saturated brine three times, dried over anhydrous sodium sulfate, and silica gel column Compound 3 was isolat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com