Method for adsorbing and separating sulfur-containing acid gas
An adsorption separation, sulfuric acid technology, applied in the field of chemical engineering, can solve the problems of limited adsorption capacity and low pore volume, and achieve the effect of high separation selectivity, low cost and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
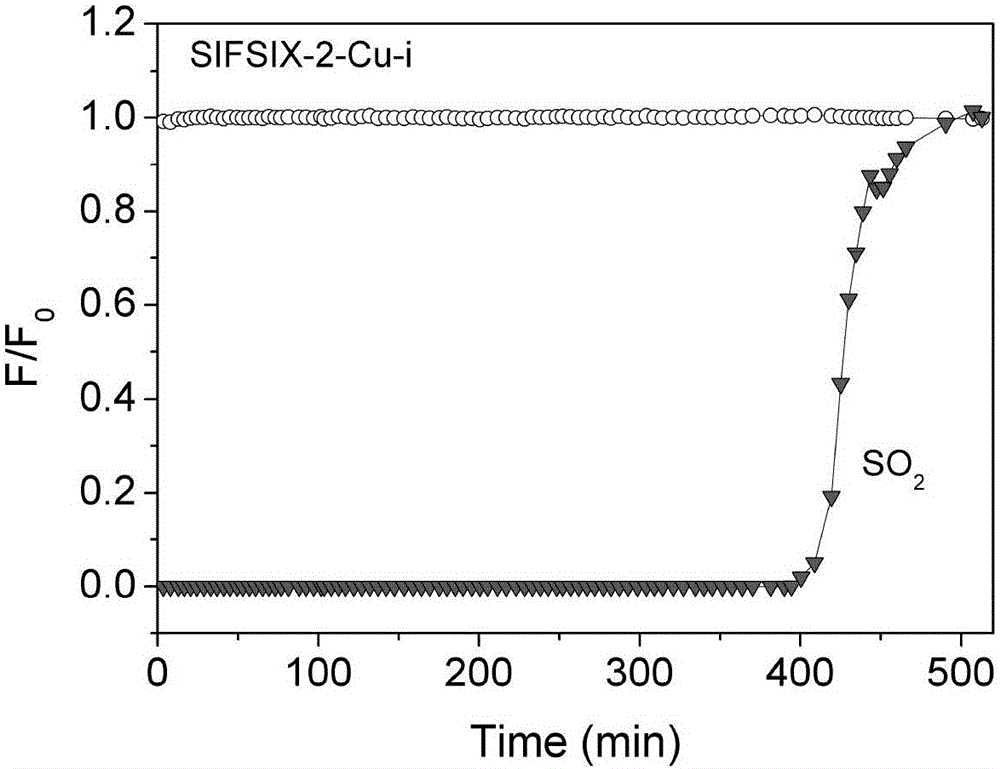
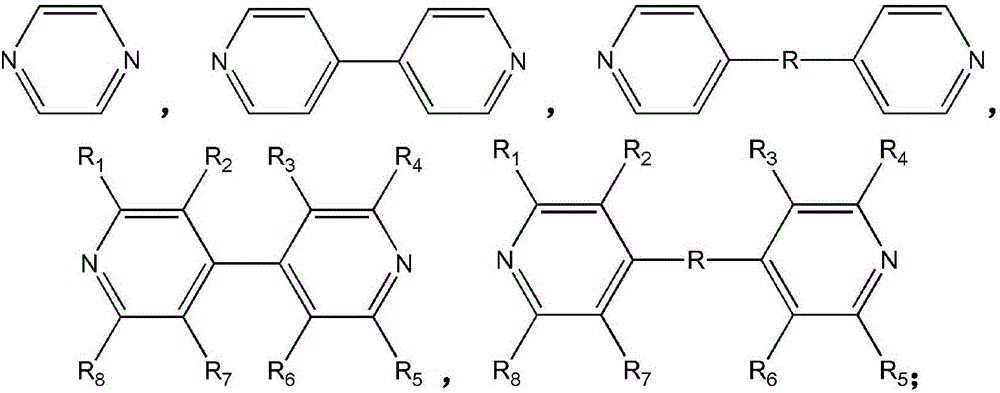
[0042] Weigh 46.44 mg of 4,4'-dipyridylacetylene (organic ligand L1) and dissolve it in 4 ml of methanol, weigh 89 mg of Cu (BF 4 ) 2 ·xH 2 O and 45.96 mg (NH 4 ) 2 SiF 6 (Inorganic anion ligand L2) was dissolved in 4ml of water, and the above two solutions were mixed under stirring, and then heated to 60-100° C. for 12-36 hours to react. The obtained product SIFSIX-2-Cu-i (the inorganic anion ligand is SiF 6 - ) was filtered, washed with methanol, and then activated. The adsorbent was packed into the adsorption column (inner diameter 4.6mm, length 50mm), and at room temperature 25°C, 2000ppm SO 2 , 99.8%N 2 The mixed gas is passed into the adsorption column at 20ml / min, and the nitrogen gas with extremely low sulfur dioxide content (2 and 99.8% N 2 The breakthrough curve of the mixed gas on SIFSIX-2-Cu-i is as follows figure 1 shown.
Embodiment 2
[0044] Example 1 obtains an adsorption column (inner diameter 4.6mm, length 50mm), and at a room temperature of 35°C, 10% SO 2 , 90% air mixed gas is passed into the adsorption column at 20ml / min, nitrogen with extremely low sulfur dioxide content (<10ppm) is obtained in the first 140min, and the adsorption is stopped. At 80°C, vacuumize and desorb sulfur dioxide gas.
Embodiment 3
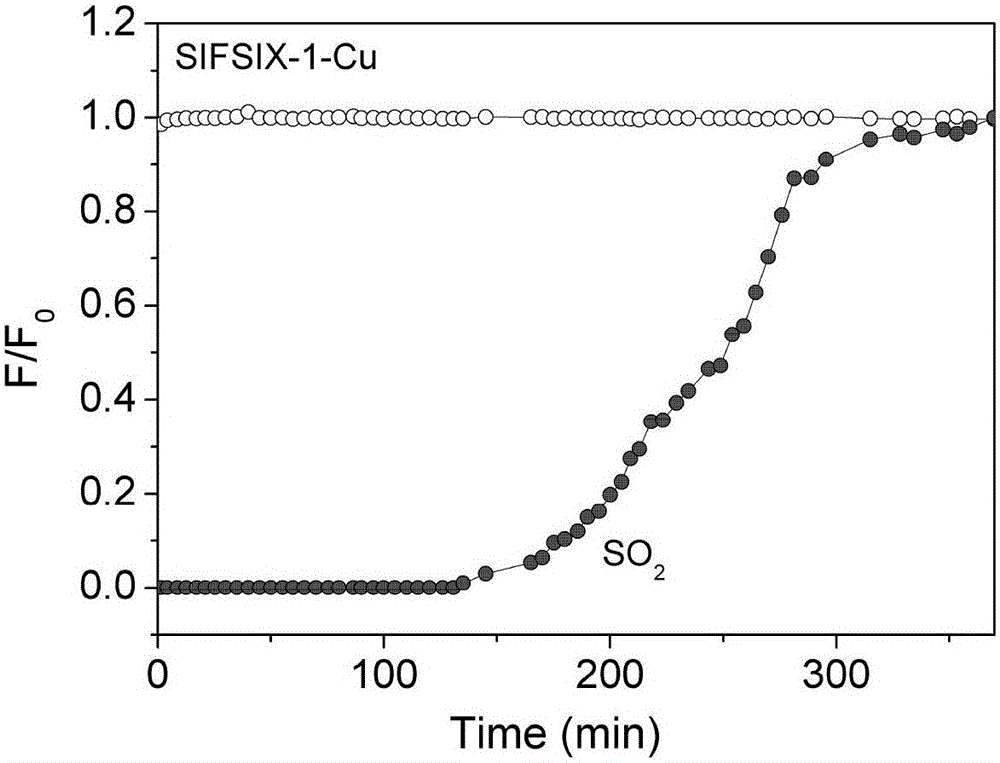
[0046] Weigh 0.35 g of 4,4'-bipyridine (organic ligand L1) and dissolve it in ethylene glycol. Weigh 0.28 g Cu(BF 4 ) 2 ·xH 2 O (metal ion M1) and 0.199 g (NH 4 ) 2 SiF 6 (Inorganic anionic ligand L2) was dissolved in deionized water and added to the 4,4'-bipyridine glycol solution. The reaction was stirred at less than 100°C for 2-8 hours. The purple powder product SIFSIX-1-Cu (the inorganic anion ligand is SiF 6 - ) filter and then activated. The adsorbent was packed into the adsorption column (inner diameter 4.6mm, length 50mm), at room temperature 25°C, 2000ppmSO 2 , 99.8% methane mixed gas is passed into the adsorption column at 20ml / min, nitrogen with extremely low sulfur dioxide content (2 and 99.8% N 2 The breakthrough curve on SIFSIX-1-Cu is as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com